the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Decoding pelagic ciliate (Ciliophora) community divergences in size spectrum, biodiversity and driving factors globally spanning five temperature zones
Chaofeng Wang
Zhiqiang Xu
Guangfu Luo
Xiaoyu Wang
Yan He
Musheng Lan
Tiancheng Zhang
The community structure of microzooplanktonic ciliates – encompassing size spectrum, biodiversity and biotic–abiotic interplay – is critical for unravelling their ecological role in marine ecosystems, yet it remains challenging to elucidate on a global scale. To address this knowledge gap, we conducted field observational studies across five temperature zones (North Frigid Zone, NFZ; Sub-Arctic Zone, SAZ; North Temperate Zone, NTZ; Torrid Zone, TZ; South Frigid Zone, SFZ). Our analysis demonstrates a sharp decline in ciliate abundance and biomass below the 100 m layer, with distinct vertical distribution patterns observed in each climate region. Moreover, although abundance of ciliate size spectra exhibited a decrease trend from small to large size spectra globally, there were steeper slope lines observed in both polar zones (NFZ and SFZ) compared to the other temperature zones. Latitudinally, ciliate abundance and tintinnid biodiversity exhibited an anti-phase relationship, where the TZ hosted peak biodiversity, while the polar seas showed the highest abundance. Furthermore, a multivariate biota–environment analysis indicated that temperature has a primary influence on ciliate community constitution in the global marine ecosystem, and the bottom-up control plays a key role in shaping assemblages. In conclusion, these results underscore the unprecedented divergences in ciliate trait structure among five temperature zones and can be taken as a guideline for assessing the potential effects of climate change on pelagic ciliates in future marine realms.
- Article
(6561 KB) - Full-text XML
-
Supplement
(8231 KB) - BibTeX
- EndNote
The Earth is traditionally partitioned into five temperature zones based on established climate classifications: the North Frigid Zone (NFZ), North Temperate Zone (NTZ), Torrid Zone (TZ), South Temperate Zone (STZ) and South Frigid Zone (SFZ) (Köppen, 1936; Trewartha et al., 1967). Therein, each temperature zone possessed a unique ocean circulation pattern and concurrent specific plankton biome structures (Longhurst, 2007; Spalding et al., 2012). Although a myriad of prevailing research exists relevant to plankton biogeography and its interplay with environmental drivers highlighting its importance in disentangling marine ecosystems and biogeochemical cycles (e.g. Wang et al., 2020; Darnis et al., 2022; Segaran et al., 2023; Tagliabue et al., 2023), substantial global-scale studies have predominantly relied on modelling frameworks (Spalding et al., 2012; Blanchard et al., 2017; Anderson et al., 2021; Benedetti et al., 2021; Heneghan et al., 2023; Atkinson et al., 2024). To date, an explicit and comprehensive representation of plankton community trait structure using data-derived statistical analysis originating from field surveys remains unresolved.
A holistic paradigm of plankton biogeography across marine ecosystem is crucial for deciphering global ecological connectivity (Hillman et al., 2018) and predicting how ecosystems respond to stressors induced by climate change (Darnis et al., 2022). Over recent decades, anthropogenic CO2 emissions have led to increased atmospheric concentrations and greater global radiative forcing (IPCC, 2023), triggering diverse ecological feedbacks worldwide, for instance poleward distribution shifts (Neukermans et al., 2018; Oziel et al., 2020; Benedetti et al., 2021), adjustments in phenology (Poloczanska et al., 2013; Atkinson et al., 2015; Chust et al., 2024) and reductions in mean body size (Daufresne et al., 2009; Verberk et al., 2021; Wang et al., 2023a, b). In this sense, extensive existing studies put emphasis on biotic community response to climate change in the polar and adjacent seas, owing to their higher susceptibility compared to tropical, subtropical and temperate seas (Serreze et al., 2009; Screen and Simmonds, 2010; IPCC, 2023; Noh et al., 2024). Unfortunately, informative research related to environmental affinity of plankton, particularly microzooplankton, is not sufficiently understood in the aforementioned five temperature zones.
In the realm of microzooplankton, pelagic ciliates stand out as the predominant biological entities, spanning in size from 10 to 200 µm, and hold significant sway over both biodiversity and abundance, particularly in the polar and adjacent seas (Taniguchi, 1984; Strom and Fredrickson, 2008; Lu and Weisse, 2022; Kohlbach et al., 2023; Wang et al., 2023b, 2024a, b). Taxonomically categorized within the phylum Ciliophora, class Spirotrichea, and subclasses Oligotrichia and Choreotrichia, pelagic ciliates, including aloricate ciliates and tintinnids, are ubiquitous single-cell protozoans found in various aquatic environments worldwide (Lynn, 2008). Furthermore, ciliates play an irreplaceable role in marine trophodynamics (carbon cycle and energy transfer) through prey–predator interactions, serving as both phytoplankton grazers and prey for metazoans (Stoecker et al., 1987; Dolan et al., 1999; Calbet and Saiz, 2005; Gómez, 2007; Weisse and Sonntag, 2016). Specifically, owing to their simple life cycle, fast reaction to environmental changes and strong adaptability, pelagic ciliates, particularly tintinnids, are widely recognized as ideal bioindicators for assessing various sea conditions (e.g. Kato and Taniguchi, 1993; Jiang et al., 2013; Wang et al., 2021; Yu et al., 2022).
Recent escalation in global warming has imposed a cascade of impacts on aquatic ecosystems, presenting a formidable challenge to inherent holopelagic species that modify their relevant adaptive strategies (Stabeno et al., 2012; Yasumiishi et al., 2020; Carvalho et al., 2021; Atkinson et al., 2024). Accordingly, a prevailing viewpoint on phytoplankton, the cornerstone of the marine pelagic food web, is a major decline in both biomass and size spectra in the NTZ, TZ and STZ (Li et al., 2009; Lotze et al., 2019; Tittensor et al., 2021), leading to subsequent declines in higher trophic levels, termed “trophic amplification” (Kwiatkowski et al., 2019; du Pontavice et al., 2021). As a grazer of pelagic phytoplankton, response of microzooplanktonic ciliates to ocean warming in the polar and adjacent seas is substantial (Li et al., 2022; Wang et al., 2022a, 2023a, b, 2024a), yet comparative assessments amid their trait structure (e.g. size spectra, biodiversity and biotic–abiotic interplay) remain unexplored to date.
Consequently, elucidating microzooplanktonic ciliate size spectra, species diversity and biotic–abiotic interplay at a global scale is critical for projecting future marine ecosystem dynamics, particularly given their unresolved role in plankton response to climate changes. Here, we propose a hypothesis that hydrographic variability is likely responsible for the observed divergence in global ciliate trait structures. By optimizing field observational data and available methods, this study aims (1) to decode adaptive strategies of microzooplanktonic ciliate to heterogeneous hydrographic conditions across temperature zones and (2) to evaluate their potential response dynamics to accelerating climate change. Given the current foreseeable rapid climate change, this study will offer a benchmark for facilitating the phenological and bioclimatic progression of microzooplankton shifts in future global marine ecosystem realms.
2.1 Study area and field sampling
Based on their latitudinal locations, field samplings of microzooplanktonic ciliates were conducted in five temperature zones (Trewartha et al., 1967): (1) the North Frigid Zone (NFZ), encompassing the Arctic Ocean, during July to August 2019 and 2023 aboard the R/V Xiang Yang Hong 1 and R/V Xue Long 2, respectively; (2) the Sub-Arctic Zone (SAZ), located in the Bering Sea, in July to August 2019 aboard the R/V Xiang Yang Hong 1; (3) the North Temperate Zone (NTZ), situated in the North Pacific, in September 2019 aboard the R/V Dong Fang Hong 3; (4) the Torrid Zone (TZ), which includes the tropical western Pacific in December 2016 and August 2017 aboard the R/V Kexue and the Indian Ocean in March 2021 aboard the R/V Xiang Yang Hong 6; and (5) the South Frigid Zone (SFZ), covering the Southern Ocean, from December 2020 to March 2021 aboard the R/V Xue Long 2 (Fig. 1). A total of 1117 samples (175 stations along 19 transects) were sampled.
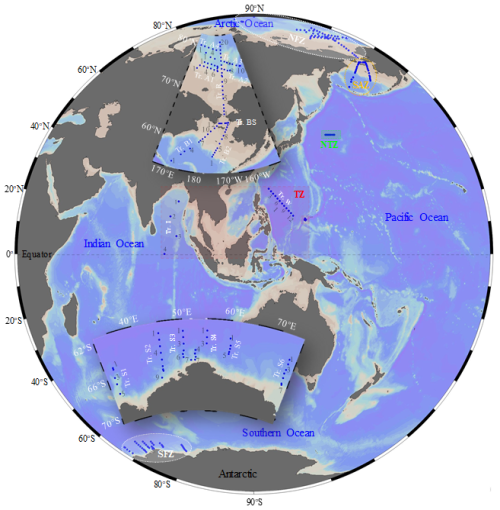
Figure 1Survey stations and transects (Tr.) in the tropical, temperate and polar seas. NFZ, North Frigid Zone; SAZ, Sub-Arctic Zone; NTZ, North Temperate Zone; TZ, Torrid Zone; SFZ, South Frigid Zone.
Seawater samples were collected with a rosette sampler carrying 24 Niskin bottles (each 12 L). All microzooplanktonic ciliate samples were collected at seven standardized depths (surface (2 m), 25, 50, 75, 100, 150 and 200 m) at each designated station, with the exception of SAZ stations, where bathymetry limited sampling to depths <200 m. Furthermore, each sample was fixed with Lugol's acid (1 % final concentration) and preserved in darkness at 4 °C until laboratory analysis.
2.2 Sample analysis
Laboratory processing involved concentrating each sample to approximately 200 mL through siphon-assisted supernatant removal following 60 h sedimentation. After two rounds of the siphon process, finally a 25 mL highly concentrated sample was obtained and then settled in a Utermöhl counting chamber (Utermöhl, 1958). Quantitative analysis was performed using an Olympus IX71 inverted microscope (100× or 400× magnification) to enumerate total ciliate abundance (including aloricate ciliates and tintinnids), measure morphometric parameters (body size) and document species richness across all five temperature zones by Chaofeng Wang. To ensure accuracy, cellular dimensions (e.g. length, width, shape) of aloricate ciliate or each tintinnid species were measured for at least 10 individuals if possible.
Additionally, the body sizes of both aloricate ciliates and tintinnids were categorized into 10 µm increments (10–20 µm, 20–30 µm, etc.) based on body length (Wang et al., 2020) and further classified into small (10–20 µm), medium (20–50 µm) and large (>50 µm) size fractions following Yang et al. (2019). Moreover, we did not distinguish the presence/absence of tintinnid lorica during the sample-counting process. Regarding species richness, tintinnid identification was assigned to the closest species, as described in Zhang et al. (2012). Furthermore, we select the average value (15, 25, 35, 45 µm etc.) of each size fraction of both loricate ciliate and tintinnid as the counting criterion for ciliate size spectra (Wang et al., 2024a). In addition, the slope or slope line means tendency of evaluating the decreasing trend from small to large size spectrum. Simultaneously, environmental factors of sampling depth (a pressure sensor to detect hydrostatic pressure, converted to depth via the formula , where ρ is water density and g is gravitational acceleration) (van Haren et al., 2021), temperature (a thermistor, SBE-3 Plus; resolution is 0.0001 °C), salinity (derived from measured electrical conductivity (SBE-4C sensor) and temperature data, computed using the practical salinity scale algorithm) and chlorophyll a in vivo fluorescence (chl a, a fluorometer [SeaPoint] excites chlorophyll pigments with blue light and measures emitted red light intensity as a proxy for chl a concentration) were recorded by a multi-sensor profiler (CTD – SeaBird SBE 911, https://www.seabird.com/product.detail-cms.block.jsa?id=60761421595, last access: 5 June 2025) during each cruise.
2.3 Data processing
Ciliate volumes were estimated according to their appropriate geometric shapes (cone, ball, cylinder). Carbon biomass of each tintinnid was calculated by the following equation (Verity and Lagdon, 1984):
where C (10−6 µg C) is the carbon biomass of individual tintinnid, and Vi (µm3) is the lorica volume. Additionally, a conversion factor ( µg C µm−3) was used for calculating aloricate ciliate carbon biomass (Putt and Stoecker, 1989). Concerning size spectra biomass, ciliate biomass was calculated based its specific organism volume and conversion equation and then categorized into each size spectrum as in Wang et al. (2024a). Furthermore, in order to better unravel tintinnid biodiversity spanning five temperature zones, the Margalef index (dMa) (Margalef, 1958) (1) and Shannon index () (Shannon, 1948) (2) were conducted by the following equations:
where S is the number of species, and N is the total number of tintinnid individuals in the sample.
where S is the number of species, and N is the total abundance of tintinnid individuals in the sample. Pi () is the relative abundance of i species in a whole community.
Biogeographically, classification of tintinnid genera (cosmopolitan, warm water, boreal, austral and neritic) was based on Pierce and Turner (1993) and Dolan and Pierce (2013). Among them, tintinnid genera were further classified into oceanic (cosmopolitan, warm water, boreal and austral) and neritic types. Moreover, the average value of each parameter is represented as mean ± SD in the following text. Finally, seasonality is important to modulate protozoan communities, but this phenomenon was obvious in both temperate and polar seas. Regarding tropic seas in both the Pacific and Indian oceans, the community structure, including vertical distribution pattern, abundance and biomass values and species composition, was almost same (e.g. Sohrin et al., 2010; Li et al., 2018; Wang et al., 2019a, 2020, 2022c).
Hereinafter, sampling map was visualized by ODV (Ocean Data View, Version 4.7), and ciliate distributional data of size–diversity and temperature–diversity relationships were analysed using Surfer (Version 13.0), Grapher (Version 12.0) and OriginPro 2021 (Version 9.6). Moreover, in order to reduce deviation in the biotic–abiotic relationship in different temperature zones that may be mainly caused by the difference in the selection of sampling areas, rather than the fundamental differences between temperature zones, the internal correlations among each temperature zone at specific sampling depth (0, 50, 100 and 200 m) were compared in the following text. Meanwhile, the biota–environment analysis was performed based on Spearman's correlation between log-transformed abiotic parameters and square root-transformed abundance data (t test) using both PRIMER (Version 5.0) and OriginPro 2021 (Version 9.6). Additionally, the slope of the size spectrum (a straight line fitted through the size spectrum on a log–log plot) (Blanchard et al., 2017) was carried out to quantize its interplay with ciliate abundance at a discrete depth of aforementioned global seas (95 % confidence). In the following, based on the slope condition, we used the decreasing rate (ΔD) or increasing rate (ΔI) according to ciliate abundance or species richness and environmental variables to quantize their interplay in the global seas.
3.1 Hydrography and ciliate abundance and biomass
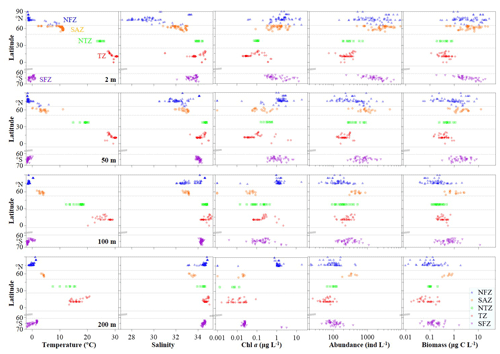
Each environmental parameter (temperature, salinity and chl a) displayed distinct spatiotemporal variations globally (Figs. 2 and S1–S3 in the Supplement). Horizontally, at the surface and 50 and 100 m layers, both temperature and salinity peaked in the Torrid Zone (TZ), contrasting with chl a, which exhibited its lowest value in the same region (Figs. 2, S1 and S2). At 200 m depth, temperature peaked in the TZ, and chl a peaked in the North Frigid Zone (NFZ), contrasting with salinity patterns, which displayed high values in both the TZ and NFZ (Figs. 2 and S1). Vertically, both temperature and chl a declined in the NFZ and Sub-Arctic Zone (SAZ) (surface-peak pattern), while salinity increased from the surface to 200 m layers across all regions (Figs. S1–S3). Moreover, temperature displayed a low–high–low structure at inner stations of the South Frigid Zone (SFZ), and chl a peaked at subsurface layers in both the North Temperate Zone (NTZ) and TZ (Fig. S1).
Pelagic ciliate abundance ranged from 22–9142 in the NFZ, 182–9242 in the SAZ, 65–886 in the NTZ, 25–436 in the TZ and 44–5866 in the SFZ, whereas their biomass ranged from 0.0–39.3 µg C L−1, 0.3–24.0 µg C L−1, 0.1–1.1 µg C L−1, 0.0–1.1 µg C L−1 and 0.0–26.1 µg C L−1 in aforementioned regions, respectively (Figs. 2 and S1–S3; Table S1 in the Supplement). Horizontally, both high abundance (≥2000 ) and biomass (≥5.0 µg C L−1) of ciliates were observed in surface layers of the NFZ, SAZ and SFZ, coinciding with high chl a levels. At 50, 100 and 200 m layers, the SAZ and TZ had the highest and lowest abundance, respectively (Figs. 2 and S1). Vertically, both ciliate abundance and biomass exhibited a surface-peak pattern in the NFZ, SAZ and SFZ, whereas in the NTZ and TZ, this pattern transitioned to subsurface-peak and bimodal-peak distributions, respectively (Figs. S1 and S2).
Meanwhile, aloricate ciliates dominated the ciliate community, accounting for ≥90 % of total abundance at each depth in the NFZ, NTZ, TZ and SFZ. However, in the SAZ, tintinnid played a more significant role in the ciliate community, with an average relative abundance at most sampling depths exceeding 10 % (Fig. S4). In terms of aloricate ciliates in the horizontal direction, small (10–20 µm) and medium (20–50 µm) size fractions in the SAZ exhibited the highest average abundance at the surface and 50, 100 and 200 m layers, whilst the largest (>50 µm) size fraction had the highest average abundance at the surface and 50 and 100 m layers in the SFZ (Fig. S5). Additionally, except for the NTZ, the abundance and relative abundance of the medium size fraction were highest in the other four regions at both the surface and 50 m layers. At 200 m depth, the small size fraction predominated among the aloricate ciliates (Figs. S5). Vertically, the relative abundance of the large size fraction (>50 µm) exhibited a decreasing trend, whereas the small size fraction displayed an increasing trend across the five temperature zones (Figs. S5).
3.2 Notable variations in pelagic ciliate size spectrum composition
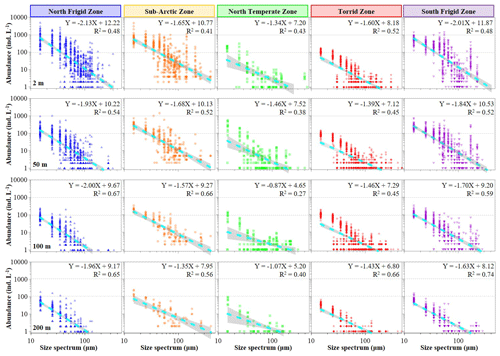
The abundance and biomass of pelagic ciliate size spectra displayed significant variations across global seas (95 % confidence) (Figs. 3 and 4). Generally, the slopes of the normalized abundance size spectra varied from −2.13 to −0.87 (average −1.60 ± 0.33), and relevant biomass values varied from −0.99 to −0.08 (average −0.53 ± 0.25), with the former slope line much steeper than the latter (Fig. 3). Therein, ciliate abundance decreased from small (15 µm) to large size spectra (>100 µm), with the slope line of the normalized abundance size spectra in both the NFZ (−2.13 to −1.93, average −2.01 ± 0.09) and SFZ (−2.01 to −1.63, average −1.80 ± 0.17) being steeper than in the other three regions at each depth (Fig. 3). Additionally, a secondary peak in abundance, featuring large size spectra (>100 µm), was observed at the surface layers of the NFZ, SAZ and SFZ (Fig. 3).
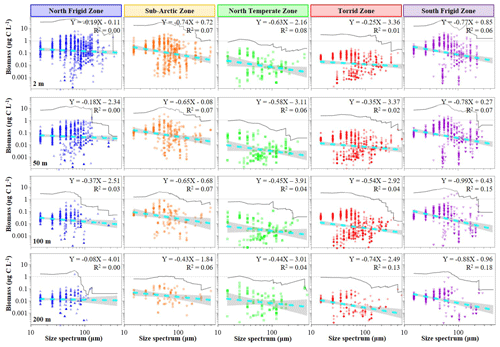
Figure 4Variations in body-size spectra of ciliate normalized biomass at discrete depth (2, 50, 100 and 200 m) in each temperature zone.
In contrast, the distribution characteristics of ciliate biomass within size spectra did not align with the abundance trend (Fig. 4). Notably, the 65 µm size spectrum exhibited the highest values at both surface and 50 m layers of the NFZ, followed by the SFZ (55 µm) and SAZ (55 µm), with the TZ (35 µm) and NTZ (25 µm) showing lower values (Fig. 4). Moreover, the slope lines of the normalized biomass size spectra in the SFZ (−0.99 to −0.77, average −0.86 ± 0.10) were steeper than those in the SAZ (−0.74 to −0.43, average −0.62 ± 0.13), NTZ (−0.63 to −0.44, average −0.53 ± 0.09), TZ (−0.74 to −0.25, average −0.47 ± 0.22) and NFZ (−0.37 to −0.08, average −0.21 ± 0.12) (Fig. 4). Interestingly, the highest biomass of ciliate size spectra at the surface and 50 and 100 m layers of the TZ corresponded to the 35 µm size spectrum, while at the 200 m layer, the 15 µm size spectrum became dominant (Fig. 4).
3.3 Dynamics in tintinnid species richness and diversity indices
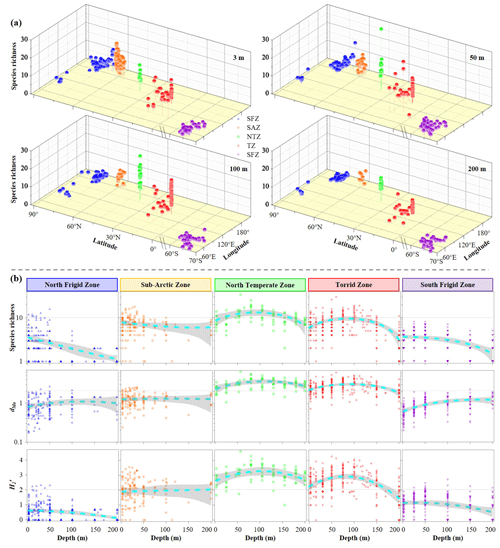
Tintinnid assemblages exhibited significant spatial heterogeneity in both species richness and diversity metrics (Margalef index dMa and Shannon index are quantitative measures of species richness in ecological communities) across five temperature zones (Figs. 5 and S6). Horizontally, species richness, Margalef index (dMa) and Shannon index () were notably high at discrete layers in both the NTZ and TZ, followed by the SAZ, NFZ and SFZ (Figs. 5a and S6). To enable cross-regional comparison, we excluded neritic genera (restricted to SAZ and NFZ) from species richness calculations, revealing higher species richness in the SFZ versus NFZ (Fig. 5a). Vertically, elevated values of tintinnid species richness, dMa and were primarily observed in the upper 50 m waters of the NFZ, SAZ and SFZ, while these values peaked at 75 and 100 m in the NTZ and TZ, respectively (95 % confidence) (Fig. 5b). Notably, we observed an inverse relationship between ciliate abundance and tintinnid species richness across five temperature zones (Fig. S7), suggesting potential competitive exclusion or niche partitioning dynamics.
3.4 Biotic–abiotic interplay and its variations
Ciliate abundance and tintinnid species richness exhibited varying correlations with environmental parameters across the five temperature zones (Figs. 6 and S8–S10). In terms of the biotic–abiotic interplay trend, our results revealed that only the NFZ and SAZ exhibited an increasing trend (ΔI≥0.03) in abundance–temperature correlation at both surface and 50 m layers compared to other three temperate zones (Fig. S9). Concerning all sampling layers, only the SFZ, differing from the trends observed in the other four temperature zones, displayed a decrease in ciliate abundance with increasing temperature (, R2=0.06) (Fig. S10). Moreover, only the TZ and SFZ exhibited an increase (ΔI≥0.29) and a decrease () trend at each sampling layer in abundance–salinity correlation, respectively (Fig. 6b). Furthermore, only SFZ showed an increase (ΔI≥0.02) trend at each sampling layer in abundance–chl a correlation (Fig. S8), which was aligned with trends in other four temperature zones at all sampling layers (ΔI≥0.06) (Fig. S10). Regarding species richness–temperature correlation, the highest increasing trend occurred at 50 m of the NFZ (ΔI=0.26, R2=0.44), while the highest decreasing trend was found at 100 m of the SAZ (, R2=0.09) (Fig. S9). As for all sampling layers, only the NFZ and TZ exhibited an increasing trend in species richness–temperature correlations, with the former (ΔI=0.15, R2=0.26) being higher than the latter (ΔI=0.06, R2=0.23) (Fig. S10). Moreover, concerning biotic–salinity correlations, only the SAZ exhibited an increasing (ΔI≥0.06) trend at each sampling layer (Fig. S9). In addition, only the polar seas exhibited an increasing trend (ΔI≥0.01) in species richness–chl a correlation at each sampling layer (Fig. S9).
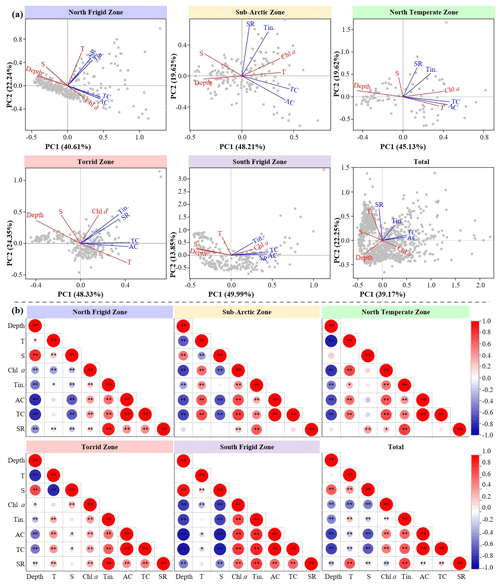
Figure 6Variations in principal component analysis (PCA) (a) and Spearman's rank correlation (b) between environmental parameters (depth; temperature, T; salinity, S; chl a) and ciliate (tintinnid, Tin; aloricate ciliate, AC; total ciliate, TC; tintinnid species richness, SR) in five regions. The x axis is the first PCA axis, and the y axis is the second PCA axis. Environmental variables and ciliates are indicated by red lines and black lines, respectively. Grey dots are sampling points. : p<0.01, ∗: p<0.05, t test.
To further quantize the physical–biological interplay in five temperature zones, we conducted both principal component analysis (PCA) and Spearman's rank correlation using abundance of aloricate ciliate, tintinnid and total ciliate, as well as tintinnid species richness to test abiotic influence (Fig. 6). The PCA revealed that two principal components effectively differentiated the environmental conditions among five temperature zones. These components accounted for a substantial proportion of the biotic variation in the NFZ (62.85 %), SAZ (67.83 %), NTZ (64.75 %), TZ (72.68 %), SFZ (63.84 %) and all regions (61.42 %) (Fig. 6a). Akin to PCA, Spearman's rank correlation reflected that abundance of aloricate ciliate, tintinnid and total ciliate in all five temperature zones displayed a strong significant negative and positive correlation with depth (p<0.01) and chl a (p<0.01), respectively (Fig. 6b). Furthermore, both aloricate ciliate and tintinnid featured a significant positive correlation with temperature in the SAZ, NTZ and TZ (p<0.05). However, in the SFZ, the relationship between aloricate ciliate and temperature shifted to a significant negative correlation (p<0.05) (Fig. 6b). Except that, tintinnid species richness exhibited a strong significant negative correlation with salinity in both the NFZ and SFZ (p<0.01), which was inconsistent with that in the NTZ, where it changed into a strong significant positive correlation (p<0.01) (Fig. 6b).
In a nutshell, this study presents a first holistic epitome of microzooplanktonic ciliate community divergences and corresponding biotic–abiotic interplay among five temperature zones (NFZ, SAZ, NTZ, TZ, SFZ) spanning the global scale, revealing significant divergence in trait-based assemblages driven by temperature zone-specific physicochemical conditions. Simultaneously, it is noteworthy that our data-driven multivariate analyses demonstrated pronounced heterogeneity in ciliate trait structures (including vertical distribution patterns, latitudinal dynamics, size spectrum and biodiversity metrics) among five temperature zones (Figs. 2–4). Among these, abiotic parameters, particularly temperature, likely played a significant role in driving these variations, as hypothesized (Chapin et al., 1997; Anderson et al., 2021; Tanioka et al., 2022; Jiao et al., 2024). However, the current dataset remains geographically constrained, particularly lacking representation from Atlantic Ocean ecosystems where ciliate communities may exhibit distinct adaptive strategies. Hence, future research should prioritize comparative studies in Atlantic systems to test the global applicability of these findings. Additionally, more emphasis should be put on uncovering the trophy mode of pelagic ciliates in marine ecosystems.
4.1 Significant divergences in functional trait of ciliate size spectrum
Plankton size spectrum, which represents the distribution of individuals within a community or ecosystem by numerical abundance or biomass across size classes typically displayed on log axes, plays a crucial role in modulating various microbial processes, such as the carbon cycle driven by prey–predator interactions (García-Comas et al., 2016; Andersen, 2019; Trombetta et al., 2020; Serra-Pompei et al., 2022; Antoni et al., 2024; Atkinson et al., 2024). Simultaneously, the size spectrum provides insights into the ecological functions within marine food webs (Vandromme et al., 2012). In this sense, although empirical evidence has elucidated both the functional traits of plankton size spectra and valuable concurrent models, the majority of integrative analyses have primarily focused on biomass density within the size spectrum rather than on the abundance distribution across different trophic levels (Sprules et al., 2016; Blanchard et al., 2017; Atkinson et al., 2024; Stukel et al., 2024). Currently, research on specific zooplankton assemblages, such as microzooplanktonic ciliates (Wang et al., 2024b), is rarely studied on a global scale. Similar to Stukel et al. (2024), our study revealed that the slopes of abundance size spectra in both the NFZ and SFZ were steeper in polar seas than the other three regions latitudinally (Fig. 3). Furthermore, the consistently steeper slopes at the surface compared to the 200 m layer across all regions (Fig. 3) suggest the following: (1) a depth-dependent shift in pelagic ciliate community size structure and (2) greater accessibility of prey for meso-/macro-zooplankton in surface waters compared to the 200 m layer, thereby influencing carbon flux efficiency to higher trophic levels (Stukel et al., 2024).
In addition, Stukel et al. (2024) depicted that the slopes of the normalized biomass size spectra varied from −1.6 to −1.2 (median slope was −1.4), spanning over 5 orders of magnitude from phytoplankton to macrozooplankton in plankton communities in the tropical and subtropical seas. In contrast, our findings revealed the median slope was about −0.53 for the biomass size spectrum (no clear straight line on a log–log plot) across all discrete depths of the global seas (Fig. 4). We deem that the finer-scale monospecific trophic group, spanning 1 order of magnitude (10–200 µm, microzooplankton), might be too small to accurately calculate the slopes of the normalized biomass size spectra (Sheldon et al., 1972). Conversely, it is noteworthy that the slopes of the abundance size spectrum exhibited an inverse relationship between abundance and body size (Fig. 3), resembling the pyramid-of-numbers concept (Elton, 1927; Trebilco et al., 2013; Blanchard et al., 2017). Hence, we posit that the slope of the abundance size spectrum may be more informative than its biomass counterpart in covering 1 order of magnitude within the plankton community.
4.2 Tintinnid biodiversity dynamics and its underlying formation mechanisms
By virtue of its critical role in regulating ecosystem processes and resource utilization efficiency, plankton species diversity plays a crucial role in marine ecosystem functioning and biogeochemical cycling (Chapin et al., 1997). Similarly, a higher functionally similar species diversity enhances stability in resistance and resilience aspects of marine ecosystem processes (Ibarbalz et al., 2019; Benedetti et al., 2021; Chust et al., 2024). Consistent with both observational and modelling studies, tintinnid biodiversity was highest in the tropical and subtropical seas and was lowest in the polar seas (Fig. 5) (e.g. Sherr et al., 1997; Dolan et al., 2014, 2016; Righetti et al., 2019; Benedetti et al., 2021; Wang et al., 2020, 2024b; Li et al., 2016, 2018, 2022). Two explanations may account for this phenomenon. On the one hand, the intrinsic mechanism is the endosymbiosis (Kutschera and Niklas, 2005). After a long-term genetic DNA exchange and evolution process driven by closely prey–predation interaction (Chen et al., 2012), more diversified phytoplankton in tropical zone (Tian et al., 2024) is probably responsible for subsequent higher tintinnid biodiversity compared to polar zones through endosymbiosis mechanism (Margulis and Sagan, 2002; Clark et al., 2023).
On the other hand, physical barriers constitute a fundamental extrinsic mechanism governing plankton biogeography (Amargant-Arumí et al., 2024; Antoni et al., 2024; Chust et al., 2024). Generally, large-scale hydrographic features, particularly oceanic gyres and distinct water masses, create biogeographic discontinuities that disrupt ecological connectivity despite physical ocean connectivity (Yang et al., 2020). These mesoscale structures establish unique ecoregions with characteristic environmental sensitivities (Longhurst, 2007), as evidenced by pronounced tintinnid community differentiation across the North Pacific Gyre, Subarctic Gyre and Beaufort Gyre systems (Wang et al., 2020). Therein, our results revealed that tintinnid biodiversity was highest in the tropical (West Pacific and Indian Ocean) and temperate (North Pacific) seas, followed by the Sub-Arctic (Bering Sea) and polar seas (Arctic Ocean and Southern Ocean around Antarctic) (Fig. 5) consistent with Wang et al. (2020), proving that plankton biogeography was deeply affected by oceanic gyres. Ultimately, elucidating biodiversity patterns across diverse temperature zones provides critical insights into microzooplankton adaptive affinity potential under climate change scenarios, particularly regarding niche conservation versus ecological plasticity in response to shifting oceanographic boundaries.
4.3 Physicochemical factors determine the habitat of microzooplankton
Hydrography habitat conditions formed by large gyres (horizontal) or water masses (vertical) are critical factors in reshuffling sophisticated species composition of the microbial food web (Lennartz et al., 2024). Conventionally, temperature can impact plankton biodiversity through regulating intrinsic temperature-dependent metabolic processes, which further determine which kind of species can live in a specific temperature environment (Archibald et al., 2022; Lukić et al., 2022; Weisse, 2024). Coincidentally, the statistically positive correlation observed between tintinnid species richness and temperature (Fig. 6) fully supports the abovementioned ecological process. In this perspective, we conclude that temperature determines organism mortality by affecting their thermal affinity within biogeochemical cycles (Knies et al., 2009; Stuart-Smith et al., 2015; Archibald et al., 2022; Chust et al., 2024) through an indirect effect (Weisse and Sonntag, 2016; Weisse, 2024). Similarly, through modulating osmotic pressure, salinity plays a crucial role in shaping the species composition of the microbial food web (Pedrós-Alió et al., 2000; Zang et al., 2024) and in hindering the dispersal of Pacific species into the Arctic Ocean (Wang et al., 2019b, 2022b). Our study, along with others, indicates that ciliate inhabiting higher salinity environments in both the TZ and NTZ (Fig. S8) compared to polar regions might be a reflection of their higher osmotic pressure affinity.
Furthermore, the chl a roughly represents the phytoplankton at a specific sampling layer, which further influences marine ecosystem stability through both quantitative (abundance) and qualitative (nutrient composition) pathways via the fundamental prey–predator interplay (Šolić et al., 2010; Våge and Thingstad, 2015; Holm et al., 2022). As direct micro-grazers of phytoplankton, both the abundance and species richness of ciliates exhibit a significant positive correlation with chl a (Figs. 6 and S8–S10), aligning with the aforementioned viewpoint regarding the ecological role of chl a (Li et al., 2024). As outlined above, coupling with our results about multivariate analyses revealed strong hydrographic–ciliate relationships (Fig. 6), while the observed trait plasticity in ciliate communities (Yu et al., 2022) further supports the predominance of bottom-up control mechanisms (resource availability, prey quality) (Lu and Weisse, 2022; Wang et al., 2023c, 2024c) over top-down regulation (predation pressure from microcrustaceans) (Power, 1992; Calbet et al., 2001; Worm and Myers, 2003) in structuring global pelagic ciliate communities.
4.4 Prediction for microzooplanktonic ciliate community to future global warming
Global warming, primarily stemming from anthropogenic CO2 emissions, has caused enduring and irreversible impacts on marine ecosystems globally, impelling a suite of threats to biodiversity and marine ecosystem, such as phenology evolution and adaptation, species poleward dispersal and body-size miniaturization (Daufresne et al., 2009; Poloczanska et al., 2013; Atkinson et al., 2015; Hastings et al., 2020; Møller and Nielsen, 2020; Yasumiishi et al., 2020; Wang and Wu, 2022; Qian et al., 2023; Wang et al., 2024a). To date, contemporary biogeographic observations have revealed marked increases in planktonic abundance and biodiversity across polar and subpolar seas (Ershova et al., 2015; Wassmann et al., 2015; Hunt et al., 2016; Kim et al., 2020; Lewis et al., 2020; Mueter et al., 2021; Wang et al., 2022a, 2023b), reflecting rapid thermal niche expansion under current warming regimes. Nevertheless, it should be mentioned that future global warming is expected to induce species extirpations by both compelling species beyond their thermal limits (Benedetti et al., 2021) and disrupting optimal survival habitats (Wang et al., 2024a).
Unfortunately, surface-dwelling ciliates (Kršinić, 1982; Wang et al., 2019a, 2023b, 2024a) are particularly vulnerable to recent, more frequent extreme temperature events, especially in tropical seas. Benedetti et al. (2021) projected a median speed of approximately 35 km per decade for the poleward shift of species dispersal under a high CO2 emission scenario by the end of this century. In this perspective, our study provides a fundamental benchmark for understanding the adaptive strategies (extirpation, dispersal or adaptation) of ciliate to rapid warming processes in global seas. Meanwhile, unlike “winner” pioneer species possessing strong adaptation abilities (Casoli et al., 2020; Boutin et al., 2023), native species characterized by lower adaptive ability, such as the Arctic endemic tintinnid species Ptychocylis urnula, may either migrate passively to new environments (Wang et al., 2022a, 2023b, 2024a) or collapsed by a combination of warming and competition (Chust et al., 2024). Moreover, combined with our results that only the NFZ and SAZ exhibited an increasing trend (ΔI≥0.03) in abundance–temperature correlation at surface layers compared with other three zones (Fig. S9), we predict that the pelagic surface–dweller ciliates in both the sub-Arctic and Arctic seas will benefit from the future global warming. Furthermore, the dynamics of the pelagic ciliate community in future trophic food webs and biogeochemical flux in the global marine ecosystem will heavily rely on how indigenous and/or intrusive species adjust to a warmer ocean state amidst multiple ecosystem stressors.
Our results provide comprehensive disparities in microzooplanktonic ciliate trait structure focused on size spectrum, biodiversity and biotic–abiotic interplay based on 1117 water samples from 175 stations across five temperature zones from the North Pole to the Southern Ocean (Antarctic). Concerning ciliate size spectrum, the slope of the normalized abundance value displayed an inverse relationship between ciliate abundance and body size, resembling a pyramid norm, while the biomass-size spectrum showed relatively smoother slopes. Additionally, tintinnid biodiversity was highest in tropical and subtropical seas and lowest in polar seas, likely influenced by endosymbiosis (intrinsic mechanism) and physical barriers (extrinsic mechanism). Furthermore, the interplay between biotic and abiotic factors manifested in temperature exerting a primary influence on the ciliate community structure. Under the current foreseeable rapid global warming process, we conjecture that bottom-up control (resource limitation) will play a more primary role through an indirect way in the global marine ecosystem.
No data sets were used in this article.
The supplement related to this article is available online at https://doi.org/10.5194/os-21-1873-2025-supplement.
Writing original draft: CW, ZX, GL, XW and TZ. Investigation: CW, ZX, XW, YH and ML. Formal analysis: CW and XW. Conceptualization: CW and WZ. Funding acquisition: CW. Project administration: WZ.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
Special thanks go to the captains and crews of R/V Xue Long 2, Xiang Yang Hong 1, Xiang Yang Hong 6, Dong Fang Hong 3 and Kexue for their great help in sampling periods during the cruises. In addition, we greatly appreciate the constructive comments by Lumi Haraguchi and the anonymous referee, which dramatically improved the quality of the manuscript.
This research was supported by the National Natural Science Foundation of China (grant nos. 42206258, 42276156 and 42176228), the Shandong Provincial Natural Science Foundation (grant no. ZR2022QD022), and the International Research Project-Dynamics and Function of Marine Microorganisms (IRP-DYF2M): insight from physics and remote sensing, CNRS-CAS.
This paper was edited by Mario Hoppema and reviewed by Lumi Haraguchi and one anonymous referee.
Amargant-Arumí, M., Müller, O., Bodur, Y., Ntinou, I., Vonnahme, T., Assmy, P., Kohlbach, D., Chierici, M., Jones, E., Olsen, L., Tsagaraki, T., Reigstad, M., Bratbak, G., and Gradinger, R.: Interannual differences in sea ice regime in the north-western Barents Sea cause major changes in summer pelagic production and export mechanisms, Prog. Oceanogr., 220, 103178, https://doi.org/10.1016/j.pocean.2023.103178, 2024.
Andersen, K. H.: Chapter 2: Size spectrum theory, in: Fish Ecology, Evolution, and Exploitation. A New Theoretical Synthesis, edited by: Andersen, K. H., Princeton University Press, 15–37, ISBN 9780691192956, 2019.
Anderson, S. I., Barton, A., Clayton, S., Dutkiewicz, S., and Rynearson, T.: Marine phytoplankton functional types exhibit diverse responses to thermal change, Nat. Commun., 12, 6413, https://doi.org/10.1038/s41467-021-26651-8, 2021.
Antoni, J., Almandoz, G., Goldsmit, J., Garcia, M., FloresMelo, X., Hernando, M., and Schloss, I.: Long-term studies on West Antarctic Peninsula phytoplankton blooms suggest range shifts between temperate and polar species, Global Change Biol., 30, e17238, https://doi.org/10.1111/gcb.17238, 2024.
Archibald, K. M., Dutkiewicz, S., Laufkötter, C., and Moeller, H. V.: Thermal responses in global marine planktonic food webs are mediated by temperature effects on metabolism, J. Geophys. Res.-Oceans, 127, e2022JC018932, https://doi.org/10.1029/2022JC018932, 2022.
Atkinson, A., Harmer, R., Widdicombe, C., McEvoy, A., Smyth, T., Cummings, D., Somerfield, P., Maud, J., and Mcconville, K.: Questioning the role of phenology shifts and trophic mismatching in a planktonic food web, Prog. Oceanogr., 137, 498–512, https://doi.org/10.1016/j.pocean.2015.04.023, 2015.
Atkinson, A., Rossberg, A. G., Gaedke, U., Sprules, G., Heneghan, R., Batziakas, S., Grigoratou, M., Fileman, E., Schmidt, K., and Frangoulis, C.: Steeper size spectra with decreasing phytoplankton biomass indicate strong trophic amplification and future fish declines, Nat. Commun., 15, 381, https://doi.org/10.1038/s41467-023-44406-5, 2024.
Benedetti, F., Vogt, M., Elizondo, U., Righetti, D., Zimmermann, N. E., and Gruber, N.: Major restructuring of marine plankton assemblages under global warming, Nat. Commun., 12, 5226, https://doi.org/10.1038/s41467-021-25385-x, 2021.
Blanchard, J. L., Heneghan, R. F., Everett, J. D., Trebilco, R., and Richardson, A. J.: From bacteria to whales: Using functional size spectra to model marine ecosystems, Trends Ecol. Evol., 32, 174–186, https://doi.org/10.1016/j.tree.2016.12.003, 2017.
Boutin, K., Gaudron, S. M., Denis, J., and Lasram, F. B. R.: Potential marine benthic colonisers of offshore wind farms in the English Channel: a functional trait-based approach, Mar. Environ. Res., 190, 106061, https://doi.org/10.1016/j.marenvres.2023.106061, 2023.
Calbet, A. and Saiz, E.: The ciliate-copepod link in marine ecosystems, Aquat. Microb. Ecol., 38, 157–167, https://doi.org/10.3354/ame038157, 2005.
Calbet, A., Landry, M., and Nunnery, S.: Bacteria-flagellate interactions in the microbial food web of the oligotrophic subtropical North Pacific, Aquat. Microb. Ecol., 23, 283–292, https://doi.org/10.3354/ame023283, 2001.
Carvalho, K. S., Smith, T. E., and Wang, S.: Bering Sea marine heatwaves: Patterns, trends and connections with the Arctic, J. Hydrol., 600, 126462, https://doi.org/10.1016/j.jhydrol.2021.126462, 2021.
Casoli, E., Mancini, G., Ventura, D., Pace, D. S., Belluscio, A., and Ardizzone, G. D.: Reteporella spp. success in the re-colonization of bare coralligenous reefs impacted by Costa Concordia shipwreck: the pioneer species you did not expect, Mar. Pollut. Bull., 161, 111808, https://doi.org/10.1016/j.marpolbul.2020.111808, 2020.
Chapin III, F. S., Walker, B. H., Hobbs, R. J., Hooper, D. U., Lawton, J. H., Sala, O. E., and Tilman, D.: Biotic control over the functioning of ecosystems, Science, 277, 500–503, https://doi.org/10.1126/science.277.5325.500, 1997.
Chen, B., Landry, M. R., Huang, B., and Liu, H.: Does warming enhance the effect of microzooplankton grazing on marine phytoplankton in the ocean?, Limnol. Oceanogr., 57, 519–526, https://doi.org/10.4319/lo.2012.57.2.0519, 2012.
Chust, G., Villarino, E., McLean, M., Mieszkowska, N., Benedetti-Cecchi, L., Bulleri, F., Ravaglioli, C., Borja, A., Muxika, I., Fernandes-Salvador, J., and Lindegren, M.: Cross-basin and cross-taxa patterns of marine community tropicalization and deborealization in warming European seas, Nat. Commun., 15, 2126, https://doi.org/10.1038/s41467-024-46526-y, 2024.
Clark, M. S., Hoffman, J., Peck, L. S., Bargelloni, L., Gande, D., Havermans, C., Meyer, B., Patarnello, T., Phillips, T., Stoof-Leichsenring, K., Vendrami, D. L., Beck, A., Collins, G., Friedrich, M. W., Halanych, K. M., Masello, J. F., Nagel, R., Noren, K., Printzen, C., Ruiz, M. B., Wohlrab, S., Becker, B., Dumack, K., Ghaderiardakani, F., Glaser, K., Heesch, S., Held, C., John, U., Karsten, U., Kempf, S., Lucassen, M., Paijmans, A., Schimani, K., Wallberg, A., Wunder, L. C., and Mock, T.: Multi-omics for studying and understanding polar life, Nat. Commun., 14, 7451, https://doi.org/10.1038/s41467-023-43209-y, 2023.
Darnis, G., Geoffroy, M., Dezutter, T., Aubry, C., Massicotte, P., Brown, T., Babin, M., Cote, D., and Fortier, L.: Zooplankton assemblages along the North American Arctic: Ecological connectivity shaped by ocean circulation and bathymetry from the Chukchi Sea to Labrador Sea, Elementa-Sci. Anthrop., 10, 1, https://doi.org/10.1525/elementa.2022.00053, 2022.
Daufresne, M., Lengfellner, K., and Sommer, U.: Global warming benefits the small in aquatic ecosystems, P. Natl. Acad. Sci. USA, 106, 12788–12793, https://doi.org/10.1073/pnas.0902080106, 2009.
Dolan, J. R. and Pierce, R. W.: Diversity and distributions of tintinnid ciliates, in: The Biology and Ecology of Tintinnid Ciliates: Models for Marine Plankton, edited by: Dolan, J. R., Agatha, S., and Coats, D. W., Wiley-Blackwell, Oxford, 214–243, https://doi.org/10.1002/9781118358092, 2013.
Dolan, J. R., Vidussi, F., and Claustre, H.: Planktonic ciliates in the Mediterranean Sea: longitudinal trends, Deep-Sea Res. Pt. I, 46, 2025–2039, https://doi.org/10.1016/S0967-0637(99)00043-6, 1999.
Dolan, J. R., Yang, E. J., Kim, T. W., and Kang, S. H.: Microzooplankton in warming Arctic: a comparison of tintinnids and radiolarians from summer 2011 and 2012 in the Chukchi Sea, Acta Protozool., 52, 101–113, https://doi.org/10.4467/16890027AP.14.010.1447, 2014.
Dolan, J. R., Yang, E. J., Kang, S. H., and Rhee, T. S.: Declines in both redundant and trace species characterize the latitudinal diversity gradient in tintinnid ciliates, ISME J., 10, 2174–2183, https://doi.org/10.1038/ismej.2016.19, 2016.
du Pontavice, H., Gascuel, D., Reygondeau, G., Stock, C., and Cheung, W.: Climate–induced decrease in biomass flow in marine food webs may severely affect predators and ecosystem production, Global Change Biol., 11, 2608–2622, https://doi.org/10.1111/gcb.15576, 2021.
Elton, C.: Animal ecology, Macmillan, New York, https://doi.org/10.5962/bhl.title.7435, 1927.
Ershova, E. A., Hopcroft, R., Kosobokova, K., Matsuno, K., Nelson, R., Yamaguchi, A., and Eisner, L.: Long-term changes in summer zooplankton communities of the western Chukchi Sea, 1945–2012, Oceanography, 28, 100–115, https://doi.org/10.5670/oceanog.2015.60, 2015.
García-Comas, C., Sastri, A. R., Ye, L., Chang, C. Y., Lin, F., Su, M., Gong, G., and Hsieh, C. H.: Prey size diversity hinders biomass trophic transfer and predator size diversity promotes it in planktonic communities, P. Roy. Soc. B-Biol. Sci., 283, 20152129, https://doi.org/10.1098/rspb.2015.2129, 2016.
Gómez, F.: Trends on the distribution of ciliates in the open Pacific Ocean, Acta Oecol., 32, 188–202, https://doi.org/10.1016/j.actao.2007.04.002, 2007.
Hastings, R. A., Rutterford, L. A., Freer, J. J., Collins, R. A., Simpson, S. D., and Genner, M. J.: Climate change drives poleward increases and equatorward declines in marine species, Curr. Biol., 30, 1572–1577, https://doi.org/10.1016/j.cub.2020.02.043, 2020.
Heneghan, R. F., Everett, J. D., Blanchard, J. L., Sykes, P., and Richardson, A. J.: Climate-driven zooplankton shifts cause large-scale declines in food quality for fish, Nat. Clim. Change, 13, 470–477, https://doi.org/10.1038/s41558-023-01630-7, 2023.
Hillman, J. R., Lundquist, C. J., and Thrush, S. F.: The challenges associated with connectivity in ecosystem processes, Front. Mar. Sci., 5, 364, https://doi.org/10.3389/fmars.2018.00364, 2018.
Holm, H. C., Fredricks, H. F., Bent, S. M., Lowenstein, D. P., Ossolinski, J. E., Becker, K. W., Johnson, W. M., Schrage, K., and Van Mooy, B.: Global Ocean lipidomes show a universal relationship between temperature and lipid unsaturation, Science, 376, 1487–1491, https://doi.org/10.1126/science.abn7455, 2022.
Hunt, G. L., Drinkwater, K. F., Arrigo, K., Berge, J., Daly, K. L., Danielson, S., Daase, M., Hop, H., Isla, E., Karnovsky, N., Wolf-Gladrow, D., Laidre, K., Mueter, F. J., Murphy, E. J., Renaud, P. E., Smith, W., Trathan, P., Turner, J., and Wolf-Gladrow, D.: Advection in polar and sub-polar environments: Impacts on high latitude marine ecosystems, Prog. Oceanogr., 149, 40–81, https://doi.org/10.1016/j.pocean.2016.10.004, 2016.
Ibarbalz, F., Henry, N., Brandao, M., Martini, S., Busseni, G., Byrne, H., Coelho, L. P., Endo, H., Gasol, J., Gregory, A., Mahe F., Rigonato, J., Royo-Llonch, M., Salazar, G., Sanz-Saez, I., Scalco, E., Soviadan, D., Zayed, A., Zingone, A., Labadie, K., Ferland, J., Marec, C., Kandels, S., Pichera., M., Dimier, C., Poulain, J., Pisarev, S., Carmichae, M., Pesant, S., Babin, M., Boss, E., Iudicone, D., Jaillon, O., Acinas, S. G., Ogata, H., Pelletier, E., Stemmann, L., Sullivan, M., Sunagawa, S., Bopp, L., de Vargas, C., Karp-Boss, L., Wincker, P., Lombard, F., Bowler, C., Zinger, L., Bork, P., Cochrane, G., Follows, M., Gorsky, G., Grimsley, N., Guidi, L., Hingamp, P., Karsenti, E., Not, F., Poulton, N., Raes, J., Sardet, C., and Sabrina, S.: Global trends in marine plankton diversity across kingdoms of life, Cell, 179, 1084–1097, https://doi.org/10.1016/j.cell.2019.10.008, 2019.
IPCC: Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, IPCC, Geneva, Switzerland, 1–169, https://doi.org/10.59327/IPCC/AR6-9789291691647, 2023.
Jiang, Y., Yang, E., Min, J., Kang, S., and Lee, S.: Using pelagic ciliated microzooplankton communities as an indicator for monitoring environmental condition under impact of summer sea-ice reduction in western Arctic Ocean, Ecol. Indic., 34, 380–390, https://doi.org/10.1016/j.ecolind.2013.05.026, 2013.
Jiao, N., Luo, T., Chen, Q., Zhao, Z., Xiao, X., Liu, J., Jian, Z., Xie, S., Thomas, H., Herndl, G., Benner, R., Gonsior, M., Chen, F., Cai, W., and Robinson, C.: The microbial carbon pump and climate change, Nat. Rev. Microbiol., 1–12, https://doi.org/10.1038/s41579-024-01018-0, 2024.
Kato, S. and Taniguchi, A.: Tintinnid ciliates as indicator species of different water masses in the western North Pacific Polar Front, Fish. Oceanogr., 2, 166–174, https://doi.org/10.1111/j.1365-2419.1993.tb00132.x, 1993.
Kim, J. H., Cho, K. H., La, H. S., Choy, E. J., and Yang, E. J.: Mass occurrence of Pacific copepods in the southern Chukchi Sea during summer: implications of the high-temperature Bering Summer Water, Front. Mar. Sci., 7, 612, https://doi.org/10.3389/fmars.2020.00612, 2020.
Knies, J., Kingsolver, J., and Burch, C. Hotter is better and broader: Thermal sensitivity of fitness in a population of bacteriophages, Am. Nat., 173, 419–430, https://doi.org/10.1086/597224, 2009.
Kohlbach, D., Goraguer, L., Bodur, Y. V., Müller, O., Amargant-Arum? M., Blix, K., Bratbak, G., Chierici, M., Dabrowska, A. M., Dietrich, U., Edvardsen, B., Garcia, L. M., Gradinger, R., Hop. H., Jones, E., Lundesgaard, O., Olsen, L. M., Reigstad, M., Saubrekka, K., Tatarek, A., Wiktor, J. M., Wold, A., and Assmy, P.: Earlier sea-ice melt extends the oligotrophic summer period in the Barents Sea with low algal biomass and associated low vertical flux, Prog. Oceanogr., 213, 103018, https://doi.org/10.1016/j.pocean.2023.103018, 2023.
Köppen, W. P.: The geographical system of climates (Das geographische system der Klimate), in: Handbook of climatology (Handbuch der Klimatologie), edited by: Köppen, W. P. and Geiger, R., Gebrüder Borntraeger, Berlin, 1–44, 1, 936.
Kršinić, F.: On vertical distribution of tintinnines (Ciliata, Oligotrichida, Tintinnina) in the open waters of the South Adriatic, Mar. Biol., 68, 83–90, https://doi.org/10.1007/BF00393145, 1982.
Kutschera, U. and Niklas, K. J.: Endosymbiosis, cell evolution, and speciation, Theor. Biosci., 124, 1–24, https://doi.org/10.1016/j.thbio.2005.04.001, 2005.
Kwiatkowski, L., Aumont, O., and Bopp, L.: Consistent trophic amplification of marine biomass declines under climate change, Global Change Biol., 25, 218–229, https://doi.org/10.1111/gcb.14468, 2019.
Lennartz, S. T., Keller, D. P., Oschlies, A., Blasius, B., and Dittmar, T.: Mechanisms underpinning the net removal rates of dissolved organic carbon in the global ocean, Global Biogeochem. Cy., 38, e2023GB007912, https://doi.org/10.1029/2023GB007912, 2024.
Lewis, K. M., Van Dijken, G. L., and Arrigo, K. R.: Changes in phytoplankton concentration now drive increased Arctic Ocean primary production, Science, 369, 198–202, https://doi.org/10.1126/science.aay8380, 2020.
Li, C., Chen, K., Sun, X., Liu, L., Ming, X., Liu, X., and Wang, B.: Summer sea ice melting enhances phytoplankton and dimethyl sulfide production, Limnol. Oceanogr., 69, 2453–2472, https://doi.org/10.1002/lno.12681, 2024.
Li, H., Xu, Z., Zhang, W., Wang, S., Zhang, G., and Xiao, T.: Boreal tintinnid assemblage in the Northwest Pacific and its connection with the Japan Sea in summer 2014, PLoS One, 11, e0153379, https://doi.org/10.1371/journal.pone.0153379, 2016.
Li, H., Zhang, W., Zhao, Y., Zhao, L., Dong, Y., Wang, C., Liang, C., and Xiao, T.: Tintinnid diversity in the tropical West Pacific Ocean, Acta Oceanol. Sin., 37, 218–228, https://doi.org/10.1007/s13131-018-1148-x, 2018.
Li, H., Xu, Z., Mou, W., Gao, L., Zu, Y., Wang, C., Zhao, Y., Zhang, W., and Xiao, T.: Planktonic ciliates in different water masses of Cosmonaut and Cooperation Seas (Indian sector of the Southern Ocean) during austral summer, Polar Biol., 45, 1059–1076, https://doi.org/10.1007/s00300-022-03057-w, 2022.
Li, W., Mclaughlin, F. A., Lovejoy, C., and Carmack, E. C.: Smallest algae thrive as the Arctic Ocean freshens, Science, 326, 539–539, https://doi.org/10.1126/science.1179798, 2009.
Longhurst, A. R.: Ecological Geography of the Sea, 2nd Edn., Academic Press, Amsterdam, 2, https://doi.org/10.1016/B978-0-12-455521-1.X5000-1, 007.
Lotze, H. K., Tittensor, D. P., Bryndum-Buchholz, A., Eddy, T. D., Cheung, W., Galbraith, E. D., Barange, M., Barrier, N., Bianchi, D., Blanchard, J. L., Bopp, L., Buchner, M., Bulman, C. M., Carozza, D. A., Christensen, V., Coll, M., Dunne, J., Fulton, E., Jennings, S., Jones, M., Mackinson, S., Maury, O., Niiranen, S., Oliveros-Ramos, R., Roy, T., Fernandes, J., Schewe, J., Shin, Y., Silva, T., Steenbeek, J., Stock, C., Verley, P., Volkholz, J., Walker, N., and Worm, B.: Global ensemble projections reveal trophic amplification of ocean biomass declines with climate change, P. Natl. Acad. Sci. USA, 116, 12907–12912, https://doi.org/10.1073/pnas.1900194116, 2019.
Lu, X. and Weisse, T.: Top-down control of planktonic ciliates by microcrustacean predators is stronger in lakes than in the ocean, Sci. Rep.-UK, 12, 10501, https://doi.org/10.1038/s41598-022-14301-y, 2022.
Lukić, D., Limberger, R., Agatha, S., Montagnes, D. J., and Weisse. T.: Thermal performance of planktonic ciliates differs between marine and freshwaters: A case study providing guidance for climate change studies, Limnol. Oceanogr. Lett., 7, 520–526, https://doi.org/10.1002/lol2.10264, 2022.
Lynn, D. H.: Ciliated Protozoa: Characterization, Classification, and Guide to the Literature, 3rd edn., Springer, Berlin, 1–455, https://doi.org/10.1007/978-1-4020-8239-9, 2008.
Margalef, R.: Information theory in ecology, Gen. Syst., 3, 36–71, 1958.
Margulis, L. and Sagan, D.: Acquiring Genomes, A theory of the origin of species, Basic Books, New York, 2002.
Møller, E. F. and Nielsen, T. G.: Borealization of Arctic zooplankton–smaller and less fat zooplankton species in Disko Bay, Western Greenland, Limnol. Oceanogr., 65, 1175–1188, https://doi.org/10.1002/lno.11380, 2020.
Mueter, F. J., Iken, K., Cooper, L., Grebmeier, J. M., Kuletz, K. J., Hopcroft, R. R., Danielson, S., Collins, R., and Cushing, D.: Changes in diversity and species composition across multiple assemblages in the eastern Chukchi Sea during two contrasting years are consistent with borealization, Oceanography, 34, 38–51, https://doi.org/10.5670/oceanog.2021.213, 2021.
Neukermans, G., Oziel, L., and Babin, M.: Increased intrusion of warming Atlantic water leads to rapid expansion of temperate phytoplankton in the Arctic, Global Change Biol., 24, 2545–2553, https://doi.org/10.1111/gcb.14075, 2018.
Noh, K. M., Oh, J. H., Lim, H. G., Song, H., and Kug, J. S.: Role of Atlantification in enhanced primary productivity in the Barents Sea, Earths Future, 12, e2023EF003709, https://doi.org/10.1029/2023EF003709, 2024.
Oziel, L., Baudena, A., Ardyna, M., Massicotte, P., Randelhoff, A., Sallée, J. B., Ingvaldsen, R. B., Devred, E., and Babin, M.: Faster Atlantic currents drive poleward expansion of temperate phytoplankton in the Arctic Ocean, Nat. Commun., 11, 1–8, https://doi.org/10.1038/s41467-020-15485-5, 2020.
Pedrós-Alió, C., Calderón-Paz, J. I., MacLean, M. H., Medina, G., Marrasé, C., Gasol, J. M., and Guixa-Boixereu, N.: The microbial food web along salinity gradients, FEMS Microbiol. Ecol., 32, 143–155, https://doi.org/10.1111/j.1574-6941.2000.tb00708.x, 2000.
Pierce, R. W. and Turner, J. T.: Global biogeography of marine tintinnids, Mar. Ecol. Prog. Ser., 94, 11–26, https://doi.org/10.3354/meps094011, 1993.
Poloczanska, E., Brown, C., Sydeman, W., Kiessling, W., Schoeman, D., Moore, P., Brander, K., Bruno, J., Buckley, L., Burrows, M., Duarte, C., Halpern, B., Holding, J., Kappel, C., O'Connor, M., Pandolfi, J., Parmesan, C., Schwing, F., Thompson, S., and Richardson, A.: Global imprint of climate change on marine life, Nat. Clim. Change, 3, 919–925, https://doi.org/10.1038/NCLIMATE1958, 2013.
Power, M. E.: Top-down and bottom-up forces in food webs: do plants have primacy, Ecology, 73, 733–746, https://doi.org/10.2307/1940153, 1992.
Putt, M. and Stoecker, D. K.: An experimentally determined carbon: volume ratio for marine “oligotrichous” ciliates from estuarine and coastal waters, Limnol. Oceanogr., 34, 1097–1103, https://doi.org/10.4319/lo.1989.34.6.1097, 1989.
Qian, C., Liu, K., Pang, M., Xu, Z., Deng, L., and Liu, H.: Hypoxia and warming take sides with small marine protists: An integrated laboratory and field study, Sci. Total Environ., 882, 163568, https://doi.org/10.1016/j.scitotenv.2023.163568, 2023.
Righetti, D., Vogt, M., Gruber, N., Psomas, A., and Zimmermann, N. E.: Global pattern of phytoplankton diversity driven by temperature and environmental variability, Sci. Adv., 5, eaau6253, https://doi.org/10.1126/sciadv.aau6253, 2019.
Screen, J. A. and Simmonds, I.: The central role of diminishing sea ice in recent Arctic temperature amplification, Nature, 464, 1334–1337, https://doi.org/10.1038/nature09051, 2010.
Segaran, T. C., Azra, M., Lananan, F., and Wang, Y.: Microbe, climate change and marine environment: Linking trends and research hotspots, Mar. Environ. Res., 189, 106015, https://doi.org/10.1016/j.marenvres.2023.106015, 2023.
Serra-Pompei, C., Ward, B., Pinti, J., Visser, A., Kiorboe, T., and Andersen, K.: Linking plankton size spectra and community composition to carbon export and its efficiency, Global Biogeochem. Cy., 36, e2021GB007275, https://doi.org/10.1029/2021GB007275, 2022.
Serreze, M. C., Barrett, A. P., Stroeve, J. C., Kindig, D. N., and Holland, M. M.: The emergence of surface-based Arctic amplification, The Cryosphere, 3, 11–19, https://doi.org/10.5194/tc-3-11-2009, 2009.
Shannon, C. E.: A mathematical theory of communication, Bell Syst. Tech. J., 27, 379–423, https://doi.org/10.1002/j.1538-7305.1948.tb01338.x, 1948.
Sheldon, R. W., Prakash, A., and Sutcliffe, W.: The size distribution of particles in the ocean, Limnol. Oceanogr., 17, 327–340, https://doi.org/10.4319/lo.1972.17.3.0327, 1972.
Sherr, E. B., Sherr, B. F., and Fessenden, L.: Heterotrophic protists in the central Arctic Ocean, Deep-Sea Res. Pt. II, 44, 1665–1673, https://doi.org/10.1016/S0967-0645(97)00050-7, 1997.
Sohrin, R., Imazawa, M., Fukuda, H., and Suzuki, Y.: Full-depth profiles of prokaryotes, heterotrophic nanoflagellates, and ciliates along a transect from the equatorial to the subarctic central Pacific Ocean, Deep-Sea Res. Pt. II, 57, 1537–1550, https://doi.org/10.1016/j.dsr2.2010.02.020, 2010.
Šolić, M., Krstulović, N., Kuspilić, G., Gladan, N., Bojanić, N., Sestanovic, S., Šantić, D., and Ordulj, M.: Changes in microbial food web structure in response to changed environmental trophic status: A case study of the Vranjic Basin (Adriatic Sea), Mar. Environ. Res., 70, 239–249, https://doi.org/10.1016/j.marenvres.2010.05.007, 2010.
Spalding, M., Agostini, V., Rice, J., and Grant, S.: Pelagic provinces of the world: A biogeographic classification of the world's surface pelagic waters, Ocean Coast. Manage., 60, 19–30, https://doi.org/10.1016/j.ocecoaman.2011.12.016, 2012.
Sprules, W. G., Barth, L. E., and Giacomini, H.: Surfing the biomass size spectrum: some remarks on history, theory, and application, Can. J. Fish. Aquat. Sci., 73, 477–495, https://doi.org/10.1139/cjfas-2015-0115, 2016.
Stabeno, P. J., Farley Jr., E., Kachel, N., Moore, S., Mordy, C., Napp, J., Overland, J., Pinchuk, A., and Sigler, M.: A comparison of the physics of the northern and southern shelves of the eastern Bering Sea and some implications for the ecosystem, Deep-Sea Res. Pt. II, 65–70, 14–30, https://doi.org/10.1016/j.dsr2.2012.02.019, 2012.
Stoecker, D. K., Michaels, A., and Davis, L.: Grazing by the jellyfish, Aurelia aurita, on microzooplankton, J. Plankton Res., 9, 901–915, https://doi.org/10.1093/plankt/9.5.901, 1987.
Strom, S. L. and Fredrickson, K. A.: Intense stratification leads to phytoplankton nutrient limitation and reduced microzooplankton grazing in the southeastern Bering Sea, Deep-Sea Res. Pt. II, 55, 1761–1774, https://doi.org/10.1016/j.dsr2.2008.04.008, 2008.
Stuart-Smith, R. D., Edgar, G., Barrett, N., Kininmonth, S., and Bates, A.: Thermal biases and vulnerability to warming in the world's marine fauna, Nature, 528, 88–92, https://doi.org/10.1038/nature16144, 2015.
Stukel, M., Décima, M., Kelly, T., Landry, M., Nodder, S., Ohman, M., Selph, K., and Yingling, N.: Relationships between plankton size spectra, net primary production, and the biological carbon pump, Global Biogeochem. Cy., 38, e2023GB007994, https://doi.org/10.1029/2023GB007994, 2024.
Tagliabue, A., Twining, B., Barrier, N., Maury, O., Berger, M., and Bopp, L.: Ocean iron fertilization may amplify climate change pressures on marine animal biomass for limited climate benefit, Global Change Biol., 29, 5250–5260, https://doi.org/10.1111/gcb.16854, 2023.
Taniguchi, A.: Microzooplankton biomass in Arctic and subarctic Pacific Ocean in summer, Mem. Natl. Inst. Polar Res. Spec. Issue, 32, 63–80, 1984.
Tanioka, T., Garcia, C., Larkin, A., Garcia, N., Fagan, A., and Martiny, A.: Global patterns and predictors of C:N:P in marine ecosystems, Commun. Earth Environ. 3, 271, https://doi.org/10.1038/s43247-022-00603-6, 2022.
Tian, S. Y., Yasuhara, M., Condamine, F. L., Huang, H. M., Fernando, A. S., Aguilar, Y. M., Pandita, H., Irizuki, T., Iwatani, T., Shin, C. P., Renema, W., and Kase, T.: Cenozoic history of the tropical marine biodiversity hotspot, Nature, 632, 343–349, https://doi.org/10.1038/s41586-024-07617-4, 2024.
Tittensor, D. P., Novaglio, C., Harrison, C., Heneghan, R., Barrier, N., Bianchi, D., Bopp, L., Bryndum-Buchholz, A., Britten, G., Büchner, M., Cheung, W., Christensen, V., Coll, M., Dunne, J., Eddy, T., Everett, J., Fernandes-Salvador, J., Fulton, E., Galbraith, E., Gascuel, D., Guiet, J., John, J., Link, J., Lotze, H., Maury, O., Ortega-Cisneros, K., Palacios-Abrantes, J., Petrik, C., du Pontavice, H., Rault, J., Richardson, A., Shannon, L., Shin, Y., Steenbeek, J., Stock, C., and Blanchard, J.: Next-generation ensemble projections reveal higher climate risks for marine ecosystems, Nat. Clim. Change, 11, 973–981, https://doi.org/10.1038/s41558-021-01173-9, 2021.
Trebilco, R., Baum, J. K., Salomon, A. K., and Dulvy, N. K.: Ecosystem ecology: size-based constraints on the pyramids of life, Trends Ecol. Evol., 28, 423–431, https://doi.org/ 10.1016/j.tree.2013.03.008, 2013.
Trewartha, G. T., Robinson, A. H., and Hammond, E. H.: The Physical Elements of Geography, in: The Elements of Weather and Climate, 24, McGraw-Hill Book Company, New York, 1967.
Trombetta, T., Vidussi, F., Roques, C., Scotti, M., and Mostajir, B.: Marine microbial food web networks during phytoplankton bloom and non-bloom periods: Warming favors smaller organism interactions and intensifies trophic cascade, Front. Microbiol., 11, 502336, https://doi.org/10.3389/fmicb.2020.502336, 2020.
Utermöhl, H.: Zur vervollkommnung der quantitativen phytoplankton Methodik, Mit. Int. Ver. Theor. Angew. Limnol., 9, 1–38, 1958.
Våge, S. and Thingstad, T. F.: Fractal hypothesis of the pelagic microbial ecosystem – can simple ecological principles lead to self-similar complexity in the pelagic microbial food web?, Front. Microbiol., 6, 1357, https://doi.org/10.3389/fmicb.2015.01357, 2015.
Vandromme, P., Stemmann, L., Garcìa-Comas, C., Berline, L., Sun, X., and Gorsky, G.: Assessing biases in computing size spectra of automatically classified zooplankton from imaging systems: A case study with the ZooScan integrated system, Methods in Oceanography, 1, 3–21, https://doi.org/10.1016/j.mio.2012.06.001, 2012.
van Haren, H., Uchida, H., and Yanagimoto, D.: Further correcting pressure effects on SBE911 CTD-conductivity data from hadal depths, J. Oceanogr., 77, 137–144, https://doi.org/10.1007/s10872-020-00565-3, 2021.
Verberk, W., Atkinson, D., Hoefnagel, K., Hirst, A., Horne, C., and Siepel, H.: Shrinking body sizes in response to warming: explanations for the temperature–size rule with special emphasis on the role of oxygen, Biol. Rev., 96, 247–268, https://doi.org/10.1111/brv.12653, 2021.
Verity, P. and Lagdon, C.: Relationships between lorica volume, carbon, nitrogen, and ATP content of tintinnids in Narragansett Bay, J. Plankton Res., 6, 859–868, https://doi.org/10.1093/plankt/6.5.859, 1984.
Wang, C., Li, H., Zhao, L., Zhao, Y., Dong, Y., Zhang, W., and Xiao, T.: Vertical distribution of planktonic ciliates in the oceanic and slope areas of the western Pacific Ocean, Deep-Sea Res. Pt. II, 167, 70–78, https://doi.org/10.1016/j.dsr2.2018.08.002, 2019a.
Wang, C., Xu, Z., Liu, C., Li, H., Liang, C., Zhao, Y., Zhang, G., Zhang, W., and Xiao, T.: Vertical distribution of oceanic tintinnid (Ciliophora: tintinnida) assemblages from the Bering sea to Arctic Ocean through Bering Strait, Polar Biol., 42, 2105–2117, https://doi.org/10.1007/s00300-019-02585-2, 2019b.
Wang, C., Li, H., Xu, Z., Zheng, S., Hao, Q., Dong, Y., Zhao, L., Zhang, W., Zhao, Y., and Xiao, T.: Difference of planktonic ciliate communities of the tropical West Pacific, the Bering Sea and the Arctic Ocean, Acta Oceanol. Sin., 39, 9–17, https://doi.org/10.1007/s13131-020-1541-0, 2020.
Wang, C., Xu, M., Xuan, J., Li, H., Zheng, S., Zhao, Y., Zhang, W., and Xiao, T.: Impact of the warm eddy on planktonic ciliate, with an emphasis on tintinnids as bioindicator species, Ecol. Indic., 133, 108441, https://doi.org/10.1016/j.ecolind.2021.108441, 2021.
Wang, C., Wang, X., Xu, Z., Hao, Q., Zhao, Y., Zhang, W., and Xiao, T.: Planktonic tintinnid community structure variations in different water masses of the Arctic Basin, Front. Mar. Sci., 8, 775653, https://doi.org/10.3389/fmars.2021.775653, 2022a.
Wang, C., Yang, M., He, Y., Xu, Z., Zhao, Y., Zhang, W., and Xiao, T.: Hydrographic feature variation caused pronounced differences of planktonic ciliate community in the Pacific Arctic Region in summer 2016 and 2019, Front. Microbiol., 13, 881048, https://doi.org/10.3389/fmicb.2022.881048, 2022b.
Wang, C., Zhao, Y., Du, P., Ma, X., Li, S., Li, H., Zhang, W., and Xiao, T.: Planktonic ciliate community structure and its distribution in the oxygen minimum zones in the Bay of Bengal (eastern Indian Ocean), J. Sea Res., 190, 102311, https://doi.org/10.1016/j.seares.2022.102311, 2022c.
Wang, C., Wang, X., Wei, Y., Guo, G., Li, H., Wan, A., and Zhang, W.: Pelagic ciliate (Ciliophora) communities in the Southern Ocean: bioindicator to water mass, habitat suitability classification and potential response to global warming, Prog. Oceanogr., 216, 103081, https://doi.org/10.1016/j.pocean.2023.103081, 2023a.
Wang, C., Wang, X., Xu, Z., Luo, G., Chen, C., Li, H., Liu, Y., Li, J., He, J., Chen, H., and Zhang, W.: Full-depth vertical distribution of planktonic ciliates (Ciliophora) and a novel bio-index for indicating habitat suitability of tintinnid in the Arctic Ocean, Mar. Environ. Res., 186, 105924, https://doi.org/10.1016/j.marenvres.2023.105924, 2023b.
Wang, C., Zhao, L., Wei, Y., Xu, Z., Zhao, Y., Zhao, Y., Zhang, W., and Xiao, T.: Insights into the structure of the pelagic microbial food web in the oligotrophic tropical Western Pacific: Examining trophic interactions and relationship with abiotic variables, Mar. Pollut. Bull., 197, 115772, https://doi.org/10.1016/j.marpolbul.2023.115772, 2023c.
Wang, C., Xu, Z., Wan, A., Wang, X., Luo, G., Bian, W., Chen, Q., Chen, X., and Zhang, W.: Diatom bloom trigger notable variations in microzooplanktonic ciliate composition, body-size spectrum and biotic-abiotic interaction in the Arctic Ocean, Environ. Res., 252, 118821, https://doi.org/10.1016/j.envres.2024.118821, 2024a.
Wang, C., Xu, Z., Wang, X., He, Y., Xu, Z., Luo, G., Li, H., Chen, X., and Zhang, W.: Insights into the pelagic ciliate community in the Bering Sea: Carbon stock, driving factors and indicator function for climate change, J. Marine Syst., 244, 103975, https://doi.org/10.1016/j.jmarsys.2024.103975, 2024b.
Wang, C., Zhao, C., Zhou, B., Xu, Z., Ma, J., Li, H., Wang, W., Chen, X., and Zhang, W.: Latitudinal pronounced variations in tintinnid (Ciliophora) community at surface waters from the South China Sea to the Yellow Sea: Oceanic-to-neritic species shift, biotic-abiotic interaction and future prediction, Sci. Total Environ., 912, 169354, https://doi.org/10.1016/j.scitotenv.2023.169354, 2024c.
Wang, Y. and Wu, C.: Rapid surface warming of the Pacific Asian Marginal Seas since the late 1990s, J. Geophys. Res.-Oceans, 127, c2022JC018744, https://doi.org/10.1029/2022JC018744, 2022.
Wassmann, P., Kosobokova, K., Slagstad, D., Drinkvvater, K., Hoperoft, R., Moore, S., Ellingsen, I., Nelson, R., Carmack, E., Popova, E., and Berge, J.: The contiguous domains of Arctic Ocean advection: Trails of life and death, Prog. Oceanogr., 139, 42–65, https://doi.org/10.1016/j.pocean.2015.06.011, 2015.
Weisse, T.: Physiological mortality of planktonic ciliates: Estimates, causes, and consequences, Limnol. Oceanogr., 69, 524–532, https://doi.org/10.1002/lno.12503, 2024.
Weisse, T. and Sonntag, B.: Ciliates in Planktonic Food Webs: Communication and Adaptive Response, in: Biocommunication of Ciliates, edited by: Witzany, G. and Nowacki, M., Springer, Cham, https://doi.org/10.1007/978-3-319-32211-7_19, 2016.
Worm, B. and Myers, R.: Meta-analysis of cod-shrimp interactions reveals top-down control in oceanic food webs, Ecology, 84, 162–173, https://doi.org/10.1890/0012-9658(2003)084[0162:MAOCSI]2.0.CO;2, 2003.
Yang, E. J., Lee, Y., and Lee, S.: Trophic interactions of micro- and mesozooplankton in the Amundsen Sea polynya and adjacent sea ice zone during austral late summer, Prog. Oceanogr., 174, 117–130, https://doi.org/10.1016/j.pocean.2018.12.003, 2019.
Yang, H., Lohmann, G., Krebs-Kanzow, U., Ionita, M., Shi, X., Sidorenko, D., Gong, X., Chen, X., and Gowan E. J.: Poleward shift of the major ocean gyres detected in a warming climate, Geophys. Res. Lett., 47, e2019GL085868, https://doi.org/10.1029/2019GL085868, 2020.
Yasumiishi, E. M., Cieciel, K., Andrews, A., Murphy, J., and Dimond, J.: Climate-related changes in the biomass and distribution of small pelagic fishes in the eastern Bering Sea during late summer, 2002–2018, Deep-Sea Res. Pt. II, 181–182, 104907, https://doi.org/10.1016/j.dsr2.2020.104907, 2020.
Yu, X., Li, X., Liu, Q., Yang, M., Wang, X., Guan, Z., Yang, J., Liu, M., Yang, E., and Jiang, Y.: Community assembly and co-occurrence network complexity of pelagic ciliates in response to environmental heterogeneity affected by sea ice melting in the Ross Sea, Antarctica, Sci. Total Environ., 836, 155695, https://doi.org/10.1016/j.scitotenv.2022.155695, 2022.
Zang, L., Liu, Y., Jiao, N., Zhong, K., Song, X., Yang, Y., Cai, L., Liu, K., Mao, G., Ji, M., and Zhang, R.: Salinity as a key factor affecting viral activity and life strategies in alpine lakes, Limnol. Oceanogr., 69, 961–975, https://doi.org/10.1002/lno.12540, 2024.
Zhang, W., Feng, M., Yu, Y., Zhang, C., and Xiao, T.: An illustrated guide to contemporary tintinnids in the world, Science Press, Beijing, 1–499, ISBN 9787030343758, 2012.