the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Contribution of dark inorganic carbon fixation to bacterial carbon demand in the oligotrophic Southeastern Mediterranean Sea
Tom Reich
Natalia Belkin
Guy Sisma-Ventura
Hagar Hauzer
Maxim Rubin-Blum
Ilana Berman-Frank
Eyal Rahav
Photosynthetically derived organic matter sinking to depth from the illuminated layers is often not sufficient to meet the energy demands of microbes in the dark ocean. This “mismatch” is especially notable in the warm and oligotrophic eastern Mediterranean Sea where the annual primary production is one of the lowest in the world's oceans. Yet its aphotic zone is considered a hotspot for microbial activity. Here, we investigated the role of photic and aphotic dark inorganic carbon fixation rates (DCF) and their contribution to bacterial carbon demand in the southeastern Mediterranean Sea during the mixed and stratified periods. Our results demonstrate that DCF rates are measurable throughout the water column (0–1750 m) and are the same order of magnitude as photosynthesis (34 vs. 45 g C m−2 yr−1, respectively). Using a carbon mass balance that considers photosynthesis, DCF and bacterial production, we show that chemoautotrophy provides ∼ 35 % of the “missing carbon” supply needed for microbial growth and activity in the aphotic layer, while other sources of dissolved organic carbon remain to be elucidated. These findings underscore the need for further research into the factors affecting DCF, its role in global carbon budgets, and its potential to enhance atmospheric inorganic carbon sequestration.
- Article
(4159 KB) - Full-text XML
- BibTeX
- EndNote
The oceans aphotic layers contain the world's largest reservoir of dissolved inorganic carbon (DIC) (Baltar et al., 2010; Burd et al., 2010; Reinthaler et al., 2010), and harbor ∼ 65 % of all prokaryotes (Whitman et al., 1998). Aphotic prokaryotes typically rely on utilization of organic matter (and carbon), fixed by photoautotrophs via photosynthesis and exported from the euphotic zone, to sustain their growth and accumulate biomass (del Giorgio and Duarte, 2002). Current estimates reveal, however, a discrepancy between the supply of particulate organic carbon from photosynthesis and the bacterial organic carbon demand (BCD) in the aphotic zones (Ducklow, 2000; Karl et al., 1988; Smith and Azam, 1992). This mismatch suggests that there are other source/s of carbon that are being utilized by aphotic microorganisms (Baltar et al., 2009; Herndl and Reinthaler, 2013). One such source, that remains relatively unexplored, involves the fixation of DIC by chemo-autotrophic microbes and its assimilation into new biomass (Baltar and Herndl, 2019). This could subsequently provide bioavailable DOC to other microbial populations at depth (Baltar et al., 2010).
DIC uptake by heterotrophic bacterioplankton is generally attributed to anaplerotic reactions (Dijkhuizen and Harder, 1984; Erb, 2011) which are metabolic pathways that replenish intermediate enzymes in the citric acid cycle by fixing CO2, but other microorganisms such as nitrifying bacteria can also fix DIC (Alonso-Sáez et al., 2010). Genomic studies on deep-sea microbial communities identified several genes and metabolic pathways that enable some microbes to thrive as chemoautotrophs on inorganic substrates (Berg et al., 2007; Hallam et al., 2006). Measurements of CO2 fixation by chemoautotrophs and heterotrophic bacterioplankton are scarce, yet substantial dark DIC fixation (DCF) rates have been reported in various oceanic settings and water masses (Swan et al., 2011; Zhou et al., 2017; La Cono et al., 2018; Alothman et al., 2023) and may be more common than previously thought (Hansman et al., 2009; Herndl et al., 2005).
The deep waters of the southeast Mediterranean Sea are characterized by higher concentrations of inorganic nutrients compared to the photic zone (e.g., ∼ 6 µmol NO3+NO2 kg−1 and ∼ 0.2 PO4 µmol kg−1; Ben-Ezra et al., 2021; Sisma-Ventura et al., 2021) and low bioavailable dissolved organic carbon (Martínez-Pérez et al., 2017; Santinelli, 2015; Santinelli et al., 2010). Despite these characteristics, the southeast Mediterranean Sea's aphotic waters are considered a hotspot for bacterial activity compared to other oceanic regimes at similar depths (Luna et al., 2012; Rahav et al., 2019). Nutrient addition bioassays and water mixing simulations suggest that aphotic prokaryotes are primarily carbon-limited (Hazan et al., 2018; Rahav et al., 2019).
Here, we report on both photic and aphotic DCF and heterotrophic bacterial production rates from 6 cruises held between 2021–2023 in the southeastern Mediterranean (bottom depth 1500–1750 m) during the mixed (winter) and stratified (summer) periods. Our results demonstrate that DCF rates cannot be neglected (contrary to past convention, Nielsen, 1952) and are within the same order of magnitude as photosynthesis or heterotrophic bacterial production (BP). We also show that DCF substantially contributes to bacterial carbon demand (BCD), therefore providing, some of the “missing carbon” supply needed for microbial growth and activity in the aphotic layer of the southeast Mediterranean Sea.
2.1 Sample collection
Seawater was collected during six seasonal cruises in the Levantine Basin, southeast Mediterranean Sea, on-board the R/V Bat-Galim between 2021–2023. Three cruises were held during the stratified period and three during the winter mixing. The mixed layer depth was calculated using a temperature difference of Δ0.3 °C (Mena et al., 2019). Two “deep” stations were sampled in each cruise; one located at the edge of the continental shelf (H05 33.00° Lat, 34.50° Lon, bottom depth ∼ 1500 m, 50 km from the coast) and the other at the edge of Israel's exclusive economic zone (H06 33.15° Lat, 34.16° Lon, bottom depth 1750 m, 90 km from shoreline). Seawater was sampled at discrete depths throughout the water column, from the surface (∼ 0.5 m) to the bottom (1500–1750 m) using Niskin bottles. Sampling depths were chosen in real-time based on measurements of conductivity, temperature, depth (CTD) (Seabird 19 Plus), chlorophyll fluorescence (Turner designs, Cyclops-7) and PAR (Sea Bird). The raw hydrological data can be freely downloaded from https://isramar.ocean.org.il/isramar2009/ (last access: 25 February 2025). Measurements included DIC (NaH14CO3) uptake under ambient light (hereafter light primary productivity, LPP) or under full dark conditions (DCF), bacterial productivity (BP) and nutrient quantification.
2.2 Nitrite and ammonium concentrations
Samples for nitrite (NO) and ammonium (NH) concentrations were collected only in the 2023 cruises. The samples were pre-filtered (0.45 µm), placed in acid-washed plastic vials, and were kept frozen at −20 °C until analysis. Nutrients were measured with a Seal Analytical AA-3 system. The limits of detection for NO and NH were 0.06 and 0.09 µM, respectively.
2.3 LPP and DCF
Seawater was collected in triplicates into transparent (for LPP measurements) or dark (for DCF) Nalgene bottles (45–250 mL) and spiked with NaH14CO3 (Perkin Elmer, specific activity 56 mCi mmol−1) following Nielsen (1952). The bottles were maintained in on-deck incubators covered with a gradient of neutral mesh simulating the irradiance intensity (no change in spectrum) at 100 %, 50 %, 10 %, 1 %, and 0.1 % of surface light intensities or under complete dark conditions (Belkin et al., 2022; Reich et al., 2024). Incubators were kept at constant ambient surface temperatures (∼ 19–20 °C in winter and ∼ 28–29 °C in the summer cruises). We acknowledge that temperature differences between surface and deeper depths may alter the LPP or DCF rates measurements, especially during the summer when the water column is stratified. While in situ measurements may offer more precise rate estimates, they are generally impractical during research cruises that involve sampling at multiple locations and times throughout the day and night. Nevertheless, preliminary comparisons between the incubation setup used here versus in situ incubations using a mooring line showed negligible differences in primary productivity, falling within the expected range of measurement variability (see also Reich et al., 2022). All the incubation bottles were spiked at sunrise and terminated after 24 h (Reich et al., 2022; Robinson et al., 2009) by filtering. the particulate matter onto GF/F filters using low vacuum pressure (<50 mmHg). Next, the excess 14C-bicarbonate was removed by fuming with 50 µL of 37 % hydrochloric acid overnight. Finally, 5 mL scintillation cocktail (ULTIMA-GOLD) was added, and the disintegrations per minute (DPM) from the particulate matter concentrated on the filters were counted using a TRI-CARB 4810 TR (Packard) liquid scintillation counter. Blank seawater spiked with NaH14CO3 was filtered immediately without incubation and the reads were subtracted from the sample's DPM. The blank DPM reads were usually negligible (<5 % of the sample's DPM). Aliquots (50 µL) from random spiked samples were placed onto new GFF filters, added with 50 µL ethanolamine and scintillation liquid, and counted immediately without incubation to account for the “added activity” of the radiolabeled working solution used. LPP was calculated as the difference between the DPM retrieved from the samples incubated under ambient light (total primary production) and the “dark” bottles. Dark or light dissolved inorganic carbon fixation was calculated based on the Bermuda Atlantic Time-series Study (BATS) protocol (https://bios.asu.edu/bats/bats-data, last access: 25 February 2025). More details can be found in Reich et al. (2024).
2.4 Bacterial production
Triplicate samples per depth (1.7 mL) were incubated in the dark with 10 nmol L−1 3H-leucine L−1 (Perkin Elmer, specific activity 123 Ci mmol−1) for 4–6 h under ambient temperature (Simon et al., 1990). The incubations were terminated with 100 µL of trichloroacetic acid (100 %), processed as described by Smith and Azam (1992), and counted using a TRI-CARB 4810 TR (Packard) liquid scintillation counter. Killed control samples containing 3H-leucine L−1 and trichloroacetic acid (without incubation) were also measured and these control sample's DPMs were subtracted from the sample's reads. A conversion factor of 3 kg C mol−1 mol−1 leucine incorporated was used, assuming an isotopic dilution of 2.0 (Simon et al., 1990).
2.5 Molecular analyses and statistics
DNA was extracted from water samples with the PowerWater kit (Qiagen, USA), using the FastPrep-24™ Classic (MP Biomedicals, USA) bead-beating to disrupt the cells (2 cycles at 5.5 m s−1, with a 5 min interval). The V4 region (∼ 300 bp) of the 16S rRNA gene was amplified from the DNA (∼ 50 ng) using the 515Fc/806Rc primers amended with relevant tags (Apprill et al., 2015; Parada et al., 2016). PCR conditions were as follows: initial denaturation at 94 °C for 45 s, 30 cycles of denaturation (94 °C for 15 s), annealing (15 cycles at 50 °C and 15 cycles at 60 °C for 20 s) and extension (72 °C for 30 s). Two annealing temperatures were used to account for the melting temperature of both forward (58.5–65.5 °C), and reverse (46.9–54.5 °C), primers.
Demultiplexed paired-end reads were processed in QIIME2 V2022.2 environment (Bolyen et al., 2019). Reads were truncated based on quality plots, checked for chimeras, merged and grouped into amplicon sequence variants (ASVs) with DADA2 (Callahan et al., 2016), as implemented in QIIME2. The amplicons were classified with Scikit-Learn classifier that was trained on Silva database v138 (16S rRNA; Glöckner et al., 2017). Mitochondrial and chloroplast sequences were removed from the 16S rRNA amplicon dataset. Downstream analyses were performed in R v4.1.1 (R Core Team, 2020), using packages Phyloseq (McMurdie and Holmes, 2013) and Ampvis2 (Andersen et al., 2018). Indicator species analyses were performed using Indic species package v1.7.9 (De Cáceres et al., 2009). Amplicon reads were deposited to the NCBI SRA archive under project number PRJNA1215023.
2.6 Bacterial respiration (BR), bacterial carbon demand (BCD) and zooplankton respiration (ZR)
BR was calculated based on the following equation and assuming an average open-ocean bacterial growth efficiency (BGE) of 20 % (Herndl and Reinthaler, 2013) similar to previous direct measurements from the Mediterranean Sea ranging from 0.21–0.29 (Zweifel et al., 1993).
BCD was then calculated as the sum of BP and BR (Gasol et al., 1998). Zooplankton respiration (ZR) and excretion were compiled from Belkin et al. (2022).
3.1 Dark and light inorganic carbon fixation rates
As expected, LPP was restricted to the photic layer with highest rates usually measured at the surface (∼ 0.5 m) that gradually decreased to reach minimum rates at the bottom of the photic layer (∼ 180 m) (Fig. 1a). Relatively low LPP values were measured during the stratified summer (∼ 0.1–0.8 µg C L−1 d−1), whereas higher rates were measured during the winter mixing period (∼ 0.2–7.4 µg C L−1 d−1) (Fig. 1a). This resulted in ∼ 10-fold higher integrated rates measured during the mixed period compared to those measured during the stratified period (Table 1), in accordance with studies from the area (Psarra et al., 2005; Reich et al., 2022; Sisma-Ventura et al., 2022b). In contrast with LPP, DCF was not restricted to the photic layer and ranged from 0 to ∼ 0.4 µg C L−1 d−1 throughout the water column (Figs. 1b, 2a), without significant differences in the absolute rates between the photic and aphotic zones (t-test, p>0.05, Fig. 2b). The observed decrease in DCF rates with depth (Fig. 2a) during the summer cruises may be partly attributed to a decline in the abundance of chemoautotrophs with depth. For example, Agogué et al,. (2008) reported a decline in archaeal amoA gene copy numbers with depth in the eastern North Atlantic. Normalizing DCF rates to chemoautotrophic microbial cell abundance (or gene copy) could reveal a different vertical pattern. Another possible explanation for the decline in DCF rates with depth may be related to the weakening flux of sinking organic matter with depth that limits the substrates that fuel DCF (discussion below). The integrated photic DCF was typically lower than the rates reported in the central and western Mediterranean Sea (La Cono et al., 2018). The aphotic DCF rates were ∼ 3.5 fold higher during the mixed than during the stratified period (Table 1, Fig. 2a).

Figure 1Spatial and temporal variability in rates of LPP (a), DCF (b), BP (c) and the contribution of DCF to bacterial carbon demand (BCD) (d) at the offshore SE Mediterranean Sea (Lat. 33.15° N, Lon. 34.16° E) between 2021–2023. BCD was calculated assuming a bacterial gross efficiency of 0.20 (Gasol et al., 1998). (Figure originates from Ocean Data View; Schlitzer, 2025.)
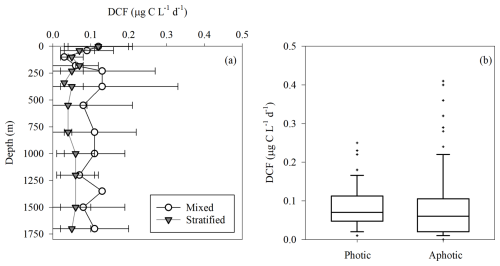
Figure 2Dark carbon fixation rates in the southeastern Mediterranean Sea. Averaged vertical distribution of DCF in the offshore southeast Mediterranean Sea during the mixed (white) and stratified (gray) periods (a), and a box plot showing the DCF rates at the photic (0–180 m) and aphotic (>180 m) water depths (b).
Table 1Depth-integrated rates and contribution of DCF to metabolic processes in the photic (0–180) and aphotic (>180 m) depths of the pelagic southeast Mediterranean Sea. The values represent the minimum and maximum ranges observed across the cruises, with the averages and their corresponding standard deviations provided in parentheses. BDL = Below detection limit.
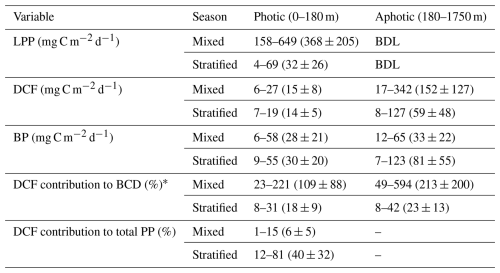
* Assuming bacterial gross efficiency of 0.2 (Gasol et al., 1998) and that the available DOC for bacteria is 20 % of the total primary productivity at the photic layer (Teira et al., 2003).
The higher aphotic DCF in the mixed versus the stratified periods may be related to more bioavailable carbon that is transported from the photic layer as marine snow and supplies organic carbon to heterotrophic activity in the winter (coinciding higher LPP). However, given the oligotrophic nature of the southeast Mediterranean Sea (Berman-Frank and Rahav, 2012; Reich et al., 2022), most of the organic carbon (both particulate and dissolved originating from LPP) is recycled within the photic layer. Only a small fraction fluxes down to the aphotic zone and has been recorded in sediment traps (Alkalay et al., 2024).
3.2 Bacterial productivity in relation to DCF and BCD
Another possible mechanism that may, potentially, explain higher DCF rates during the winter versus the summer is anaplerosis. The extent of anaplerotic reactions is primarily driven by the availability of labile organic carbon to heterotrophs (Dijkhuizen and Harder, 1984). Therefore, assuming anaplerosis drives DCF, we expect it will be positively coupled to BP.
Yet, our results do not support the likelihood of significant anaplerosis reactions. This is predominantly evident from the spatiotemporal distribution of aphotic BP (Fig. 1c) differing considerably from that of the DCF (Fig. 1b) and does not correlate with it (Fig. 3a). In fact, BP seems to be coupled with LPP at the photic layer reaching ∼ 0.4 µg C L−1 d−1 (not shown). Excluding some sporadic measurements, aphotic BP rates were usually of similar magnitude and typically <0.1 µg C L−1 d−1 (Fig. 1c). Moreover, the highest integrated aphotic BP was measured during the summers of 2021 and 2022 and not during the winter cruises when generally higher DCF was recorded (Table 1, Fig. 2a).
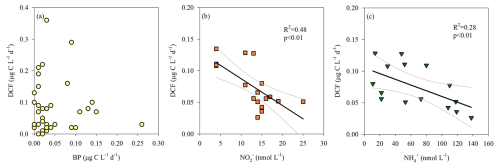
Figure 3The relationship between aphotic DCF and BP (a), NO (b) and NH (c). Note that NO and NH was measured only during the 2023 cruises. The 95 % confidence interval is shown in gray.
Despite the lack of a clear positive relationship between DCF and BP, DCF may contribute to bacterial carbon demand (BCD) in the aphotic zone. Thus, we use a literature standard, a bacterial growth efficiency of 0.20 (Gasol et al., 1998) to calculate BR and BCD (see the “material and methods” section for more details). This calculation yielded bacterial respiration (BR) ranging from 29–494 mg C m−2 d−1 (average 209 ± 172 mg C m−2 d−1), and the concurrent BCD ranges from 36 to 648 mg C m−2 d−1 (average 262 ± 121 mg C m−2 d−1). Under these circumstances, exudation of DOC from primary productivity at the photic layer estimated as 20 % of the rates (Teira et al., 2003) equals to ∼ 1–130 mg C m−2 d−1.
This new DOC, that originated from the photic zone therefore cannot support the aphotic BCD in our system in all of our observations. However, if we consider the contribution of DOC produced by aphotic DCF, part of the missing carbon may be accounted for. Thus, when considering aphotic DCF in addition to the sequestered DOC from the photic layer, the “abnormally high” aphotic BCD could be explained in full (≥100 %) in ∼ 35 % of the observations (Fig. 1d). In the other 65 % of the observations the missing carbon sources needed to support the aphotic BCD remain an enigma. We note that these calculations are based on global averages and assumptions and therefore may be subject to some uncertainties. For example, BGE can vary between seasons and sites (del Giorgio and Cole, 1998). In the Mediterranean Sea, long-term measurements of BGE ranged from 0.21 (similar to our calculations and the global average used by Herndl and Reinthaler, 2013) to 0.29 (Zweifel et al., 1993). If the 0.29 value is used, the contribution of DCF to the aphotic BCD increases to ∼ 45 % of the observations rather than ∼ 35 % when using BGE of 0.2. Similarly, if we apply an exported DOC estimate of ∼ 4 % from the photic zone, as reported for the Ionian Sea/western Mediterranean (Moutin and Raimbault, 2002), the relative contribution of DCF to aphotic BCD would be even higher than in our current calculations, which assume ∼ 20 % DOC export (Teira et al., 2003). These uncertainties warrant future investigation. Yet, even when using conservative estimates for BGE and DOC export as done here, the contribution of DCF to aphotic BCD remains substantial.
Evidence suggests that dissolved methane may be more abundant in oxygenated environments than previously thought (Grossart et al., 2011). Methane can potentially be one of the missing energy sources for marine microbes and support high BCD (Brankovits et al., 2017) as observed at the aphotic southeast Mediterranean Sea (Fig. 1d). In agreement, methanotrophs were found in aphotic cold seeps at the southeast Mediterranean Sea (Sisma-Ventura et al., 2022a), as well as across the aphotic water column in our samples (see discussion below).
3.3 Interannual variability in aphotic DCF
Interannual variability in DCF, but not in LPP or BP, was observed with higher rates recorded in March 2021–March 2022 and lower rates observed in August 2022–August 2023 (Fig. 1b). Inorganic nutrients such as PO or are unlikely to explain this variability as their ambient levels were similar between periods (https://isramar.ocean.org.il/isramar2009/, last access: 25 February 2025). Alternatively, we surmise that differences in the bioavailability and concentration of sinking organic particles, possibly attributed by the BiOS (Bimodal Oscillating System) oscillation circulation of deep water between the Adriatic and Ionian seas, could potentially explain the higher aphotic chemoautotrophic activity in March 2021–March 2022 versus August 2022–August 2023. This mechanism is known to influence the bioavailability of organic nutrients in the deep Mediterranean Sea by modulating deep-water circulation and ventilation patterns (Civitarese et al., 2010). These shifts affect the transport and residence time of organic matter (Civitarese et al., 2023), thereby potentially altering availability of organic nutrients to aphotic microbial populations, including to chemoautotrophs. Supporting this hypothesis are recent studies from the northern Red Sea and South China Sea showing that DCF is limited by labile organic nutrients such as phosphonates and even carbon-rich molecules (Reich et al., 2024; Zhou et al., 2017). Aphotic free-living chemoautotrophs are likely to encounter an increasingly refractory pool of dissolved organic matter for metabolism that may result in lower DCF rates, as shown in exported material through the water column (Santinelli et al., 2013). Particle-attached chemoautotrophs may have access to higher concentrations of organic substrates. Therefore we surmise these microbes would preferentially have a particle-attached lifestyle in the deep ocean. The patchy nature of particulate matter sinking and lateral transport during wintertime (Alkalay et al., 2024) and aggregate concentrations (Bar-Zeev et al., 2012) in the deep southeast Mediterranean Sea could also potentially explain the interannual variability in DCF between periods. Understanding how chemoautotrophs transform labile dissolved organic matter into refractory dissolved organic matter, which is an essential process in the “microbial carbon pump”, is crucial as it influences the efficiency of the biological pump (Jiao et al., 2010).
Oxidizing reduced inorganic compounds as electron donors (e.g., NO or NH) may provide chemoautotrophic prokaryotes sufficient energy to fix DIC (Hügler and Sievert, 2011). We therefore measured the vertical distribution of NO and NH (only in the March and August 2023 cruises) and examined if these chemical species are coupled or uncoupled with DCF at the aphotic zone. Our results show a negative-linear relationship between DCF and NO (Fig. 3b) and NH (Fig. 3c), suggesting nitrification. This is because chemoautotrophs consume NO and NH during nitrification to yield energy to fix DIC in the aphotic zone (REF), thereby reducing nutrient standing stocks in the water. We surmise that this “depletion” may, theoretically, explain the observed negative correlation between nutrient levels and chemoautotrophic activity. In agreement, both ammonia and nitrite oxidizers were found in the aphotic zone of all cruises (DNA level, discussion below), further highlighting their potential role as contributors to DCF in the southeast Mediterranean Sea. Nitrification measurements along with metagenomic tools, DCF (and BP) in aphotic water should be included in future dedicated studies to better refute or reinforce that oxidation of NO or NH may provide chemoautotrophic prokaryotes the energy to fix DIC.
3.4 Potential chemoautotrophs based on microbial community structure
Analyses of 16S rRNA gene amplicons suggest that diverse bacteria and archaea may drive DCF in the aphotic southeast Mediterranean Sea (Fig. 4a). Microbes found in our collected genetic material primarily include the order Nitrosopumilales ammonia-oxidizing archaea, which become dominant below DCM (up to ∼ 30 % read abundance near the bottom), corresponding to previous estimates based on in-situ fluorescent hybridization (De Corte et al., 2009). Nitrite-oxidizing Nitrospirales comprised ∼ 1 % read abundance at depths below 300 m. Among these lineages, analyses of indicator species identified seasonal variation in abundance of the orders Nitrosopumilales and Nitrososphaeria that were more prominent in the stratified period than in wintertime (p-value < 0.05) when the water column is mixed, while the deep-sea community in general exhibited only mild seasonal changes (Fig. 4b). These ammonia oxidizers may thus drive ammonia depletion during summertime at the southeast Mediterranean Sea.
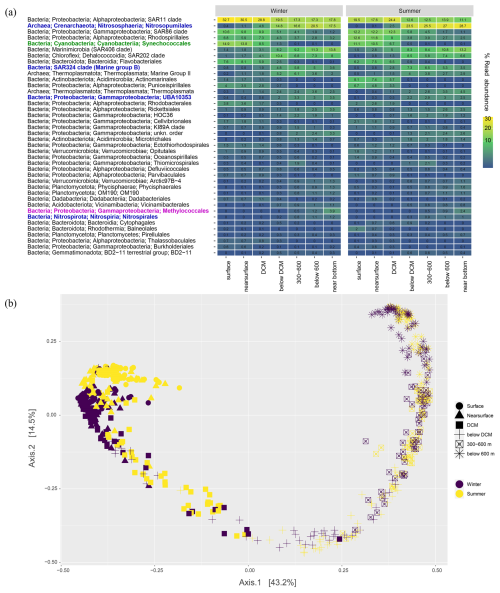
Figure 4(a) Read abundance of the 40 most abundant taxa (order level) from different depths offshore the Southeastern Mediterranean Sea. Surface samples are within the range of 1–5 m depth; near surface are between 20 and deep chlorophyll maximum (DCM); below DCM corresponds to 180–240 m depths; near bottom samples were taken circa 5 m above the seafloor. Potential DCF microbes are shown in blue, Methylococcales methanotrophs are marked in magenta, and photosynthetic Synechoccocales are shown in green for reference. (b) A principal coordinates analysis showing the differences in the structure of microbial populations based on 16S rRNA gene read mapping.
Additionally, we identified consistent occurrence of UBA10353 (Arenicellales, including the UBA868 group) and SAR324 gammaproteobacterial groups at depths below the deep chlorophyll maxima (Fig. 4a). Members from these ubiquitous clades are mixotrophs that can fix inorganic carbon conserving energy from sulfur oxidation (Baltar et al., 2023; Jaffe et al., 2024; Swan et al., 2011). We did not find the SUP05/Arctic96BD-19 gammaproteobacterial sulfur oxidizers that occur in productive dark oxygenated waters (Swan et al., 2011). We note that Methylococcales methane oxidizers were typical in the near-bottom water layer, as found previously in the southeast Mediterranean Sea basin (Sisma-Ventura et al., 2022a; Techtmann et al., 2015), suggesting methylotrophy as a potential mechanism of 1-carbon molecule acquisition in the dark southeast Mediterranean Sea. While this DNA-based community analysis provides insight into the potential contributors to DCF, it does not reflect a direct link as we cannot determine which of the identified microbes are responsible for the measured DCF rates. We stress that future studies should examine the link between DCF and specific microbial groups such as archaea by targeting RNA-level expression and functional genes (e.g., amoA and amoB), as demonstrated by Agogué et al. (2008).
Based on the conceptual model in Fig. 5 that summarizes the annual microbial carbon exchanges in the southeast Mediterranean sea's offshore area, DOC supplied by LPP is negligible and cannot explain the “high” BCD in the area, especially in the aphotic zone that is considered a “microbial hotspot” with relatively high bacterial activity per cell (Hazan et al., 2018; Rahav et al., 2019). Our observations suggest that DCF may provide a substantial amount of the missing carbon, at least in the southeast Mediterranean Sea, while source/s for the remaining missing carbon are currently unknown and warrant more research.
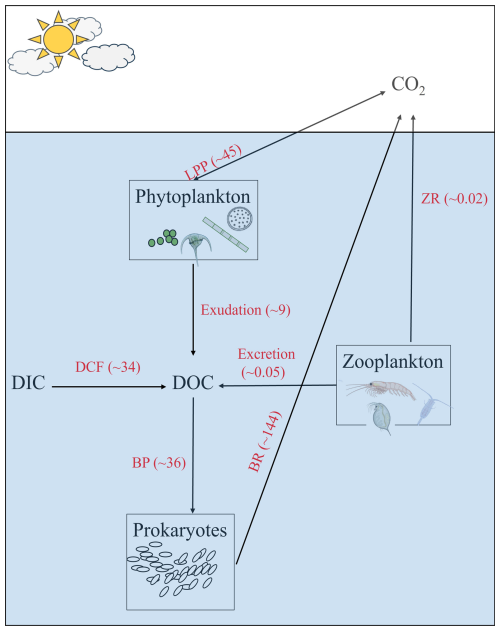
Figure 5A schematic illustration showing microbial carbon exchange in the southeast Mediterranean Sea. Values shown are the annual averages of LPP, DCF and BP. DOC exudation from LPP was assumed to be 20 % of the rates, BR was calculated from BP and assuming BGE = 0.20, Zooplankton's respiration (ZR) and excretion were compiled from Belkin et al. (2022). The numbers in brackets show the depth-integrated values over 1750 m and expressed as g C m−2 yr−1.
Regardless of the yet missing DOC sources, our results demonstrate the pivotal role DCF plays in compensating for metabolic imbalances in carbon sources at the aphotic southeast Mediterranean Sea. Similarly, DCF was shown to be a significant process supporting microbial respiration and/or activity in aphotic layers (Baltar et al., 2009; Herndl et al., 2005; Yakimov et al., 2011), as well as in hydrothermal vents (Mattes et al., 2013) and cold seeps (Nakagawa et al., 2007). Note that, while our estimates of DCF contribution to aphotic BCD are based on widely accepted assumptions, they are subject to some uncertainties, particularly regarding BGE that may be changed on both spatial and temporal scales, as well as the fraction of DOC exported from the photic zone that may also change between seasons and water provinces (see discussion below). These uncertainties underscore the need for more precise and region-specific measurements of BGE and DOC fluxes to better constrain the role of DCF in deep ocean carbon cycling.
Another potential uncertainty in measuring aphotic metabolic rates such as DCF or BP lies in the unclear effects of hydrostatic pressure on the activity of bulk microbial communities (Riebesell et al., 2009; Tamburini et al., 2013). Laboratory-based manipulations that do not account for in situ pressure conditions may alter DCF rates, potentially misrepresenting the actual contribution of chemoautotrophs to aphotic BCD. This highlights the urgent need for more detailed investigations into how hydrostatic pressure influences microbial activity in the deep ocean.
Additionally, despite the contribution of DCF to the DOC pool (taking into account the uncertainties associated with it discussed above), as well as the other sources, very little fixed carbon as particulate organic matter (POC) ends up in sediment traps located above the seabed (2 %–6 %). This suggests that most of the fixed carbon arriving from DCF (as well as LPP other potential sources) is recycled in the water column and does not reach the seabed. The rapid microbial recycling of nutrients was mostly investigated in the photic layer of the southeast Mediterranean Sea (e.g., PO4, Thingstad et al., 2005) and little is known about the processes, which are often cryptic (e.g., NO oxygenation), occurring in the aphotic layers.
Understanding the “dark end” of the biological pump in oligotrophic oceans, which plays an important (yet variable) role in oceanic carbon cycling and sequestration, will require a multidisciplinary approach that takes into account all the uncertainties discussed above in light of our (and others) observations of DCF, especially in the context of ongoing and significant changes in the marine environment. Our study supports the need for adding DCF measurements to global carbon budgets as also mentioned by Baltar et al. (2010).
The raw physiological data used to generate Figs. 1–3 can be downloaded from the PANGEA repository: https://doi.org/10.1594/PANGAEA.975231 (Reich, 2025). Hydrological data can be downloaded from https://isramar.ocean.org.il/isramar2009/ (last access: 3 February 2025). Amplicon sequencing data are available as NCBI SRA archive project number PRJNA1215023.
TR: Formal experimental work and data analyses; Investigation; Data Curation; Visualization; Writing – Original Draft Preparation. NB: Resources; Writing – Review & Editing. GSV: Investigation; Writing – Review & Editing. HaH: Investigation. MRB: Investigation; Writing – Review. IBF: Conceptualization; Funding Acquisition; Supervision; data interpretation, Writing – Review & Editing. ER: Conceptualization; Formal Analysis; Funding Acquisition; Supervision; Visualization; Writing – Review & Editing.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Also, please note that this paper has not received English language copy-editing. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
We would like to thank the University of Haifa for supporting T. Reich. This project was supported by the Helmholtz International Laboratory – a joint project of the University of Haifa (Israel) and GEOMAR Helmholtz Centre for Ocean Research (Kiel, Germany): The Eastern Mediterranean Sea Centre- An Early-Warning Model-System for our Future Oceans: EMS Future Ocean REsearch (EMS FORE) and the Captain and Crews of the R/V Bat Galim and any other people who helped.
This study is in partial fulfillment of the PhD requirements for T. Reich at the Leon H. Charney School of Marine Sciences, at the University of Haifa.
This work was supported by the German-Israeli Foundation for Scientific Research and Development (grant no. 2016021) and The National Monitoring Program of Israel's Mediterranean Waters. This work was partialy supported by IBF lab and a PhD Fellowship (to TR) was provided by the Helmholtz International Laboratory - a joint project of the U. of Haifa (Israel) and GEOMAR Helmholtz Centre for Ocean REsearch (Kiel, Germany): The Eastern Mediterranean Sea Centre - An Early Warning Model System for our Future Oceans(EMS-FORE) and by The National Monitoring Program of Israel's Mediterranean Waters through Rahav Lab.
This paper was edited by Damian Leonardo Arévalo-Martínez and reviewed by two anonymous referees.
Agogué, H., Brink, M., Dinasquet, J., and Herndl, G. J.: Major gradients in putatively nitrifying and non-nitrifying Archaea in the deep North Atlantic, Nature, 456, 788–792, https://doi.org/10.1038/nature07535, 2008.
Alkalay, R., Weinstein, Y., Herut, B., Ozer, T., Zlatkin, O., Bar, T., Berman-Frank, I., and Katz, T.: Temporal Pattern and Profile of a Coastal-Deep Sea Conveyor at a Marginal Deep Oligotrophic Sea, J. Geophys. Res. Oceans, 129, https://doi.org/10.1029/2023JC020441, 2024.
Alonso-Sáez, L., Galand, P. E., Casamayor, E. O., Pedrós-Alió, C., and Bertilsson, S.: High bicarbonate assimilation in the dark by Arctic bacteria, ISME Journal, 4, 1581–1590, https://doi.org/10.1038/ismej.2010.69, 2010.
Alothman, A., López-Sandoval, D., Duarte, C. M., and Agustí, S.: Bacterioplankton dark CO2 fixation in oligotrophic waters, Biogeosciences, 20, 3613–3624, https://doi.org/10.5194/bg-20-3613-2023, 2023.
Andersen, K. S., Kirkegaard, R. H., Karst, S. M., and Albertsen, M.: ampvis2: an R package to analyse and visualise 16S rRNA amplicon data, bioRxiv [preprint], https://doi.org/10.1101/299537, 2018.
Apprill, A., Mcnally, S., Parsons, R., and Weber, L.: Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton, Aquatic Microbial Ecology, 75, 129–137, https://doi.org/10.3354/ame01753, 2015.
Baltar, F. and Herndl, G. J.: Ideas and perspectives: Is dark carbon fixation relevant for oceanic primary production estimates?, Biogeosciences, 16, 3793–3799, https://doi.org/10.5194/bg-16-3793-2019, 2019.
Baltar, F., Arístegui, J., Gasol, J. M., Sintes, E., and Herndl, G. J.: Evidence of prokaryotic metabolism on suspended particulate organic matter in the dark waters of the subtropical North Atlantic, Limnol. Oceanogr., 54, 182–193, https://doi.org/10.4319/lo.2009.54.1.0182, 2009.
Baltar, F., Arístegui, J., Sintes, E., Gasol, J. M., Reinthaler, T., and Herndl, G. J.: Significance of non-sinking particulate organic carbon and dark CO2 fixation to heterotrophic carbon demand in the mesopelagic northeast Atlantic, Geophys. Res. Lett., 37, https://doi.org/10.1029/2010GL043105, 2010.
Baltar, F., Martínez-Pérez, C., Amano, C., Vial, M., Robaina-Estévez, S., Reinthaler, T., Herndl, G. J., Zhao, Z., Logares, R., Morales, S. E., and González, J. M.: A ubiquitous gammaproteobacterial clade dominates expression of sulfur oxidation genes across the mesopelagic ocean, Nat. Microbiol., 8, 1137–1148, https://doi.org/10.1038/s41564-023-01374-2, 2023.
Bar-Zeev, E., Berman-Frank, I., Girshevitz, O., and Berman, T.: Revised paradigm of aquatic biofilm formation facilitated by microgel transparent exopolymer particles, Proc. Natl. Acad. Sci. USA, 109, 9119–9124, https://doi.org/10.1073/pnas.1203708109, 2012.
Belkin, N., Guy-Haim, T., Rubin-Blum, M., Lazar, A., Sisma-Ventura, G., Kiko, R., Morov, A. R., Ozer, T., Gertman, I., Herut, B., and Rahav, E.: Influence of cyclonic and anticyclonic eddies on plankton in the southeastern Mediterranean Sea during late summertime, Ocean Sci., 18, 693–715, https://doi.org/10.5194/os-18-693-2022, 2022.
Ben-Ezra, T., Krom, M. D., Tsemel, A., Berman-Frank, I., Herut, B., Lehahn, Y., Rahav, E., Reich, T., Thingstad, T. F., and Sher, D.: Deep-Sea Research Part I Seasonal nutrient dynamics in the P depleted Eastern Mediterranean Sea, Deep-Sea Research Part I, 176, 103607, https://doi.org/10.1016/j.dsr.2021.103607, 2021.
Berg, I. A., Kockelkorn, D., Buckel, W., and Fuchs, G.: A 3-hydroxypropionate/4-hydroxybutyrate autotrophic carbon dioxide assimilation pathway in archaea, Science, 318, 1782–1786, https://doi.org/10.1126/science.1149976, 2007.
Berman-Frank, I. and Rahav, E.: Dinitrogen fixation as a source for new production in the Mediterranean Sea: A review, in: Life in the Mediterranean Sea, Nova Science Publishers, Inc., United States, 199–226, ISBN 9781612096445, 2012.
Bolyen, E., Rideout, J. R., Dillon, M. R., Bokulich, N. A., Abnet, C. C., Al-Ghalith, G. A., Alexander, H., Alm, E. J., Arumugam, M., Asnicar, F., Bai, Y., Bisanz, J. E., Bittinger, K., Brejnrod, A., Brislawn, C. J., Brown, C. T., Callahan, B. J., Caraballo-Rodríguez, A. M., Chase, J., Cope, E. K., Da Silva, R., Diener, C., Dorrestein, P. C., Douglas, G. M., Durall, D. M., Duvallet, C., Edwardson, C. F., Ernst, M., Estaki, M., Fouquier, J., Gauglitz, J. M., Gibbons, S. M., Gibson, D. L., Gonzalez, A., Gorlick, K., Guo, J., Hillmann, B., Holmes, S., Holste, H., Huttenhower, C., Huttley, G. A., Janssen, S., Jarmusch, A. K., Jiang, L., Kaehler, B. D., Kang, K. Bin, Keefe, C. R., Keim, P., Kelley, S. T., Knights, D., Koester, I., Kosciolek, T., Kreps, J., Langille, M. G. I., Lee, J., Ley, R., Liu, Y. X., Loftfield, E., Lozupone, C., Maher, M., Marotz, C., Martin, B. D., McDonald, D., McIver, L. J., Melnik, A. V., Metcalf, J. L., Morgan, S. C., Morton, J. T., Naimey, A. T., Navas-Molina, J. A., Nothias, L. F., Orchanian, S. B., Pearson, T., Peoples, S. L., Petras, D., Preuss, M. L., Pruesse, E., Rasmussen, L. B., Rivers, A., Robeson, M. S., Rosenthal, P., Segata, N., Shaffer, M., Shiffer, A., Sinha, R., Song, S. J., Spear, J. R., Swafford, A. D., Thompson, L. R., Torres, P. J., Trinh, P., Tripathi, A., Turnbaugh, P. J., Ul-Hasan, S., van der Hooft, J. J. J., Vargas, F., Vázquez-Baeza, Y., Vogtmann, E., von Hippel, M., Walters, W., Wan, Y., Wang, M., Warren, J., Weber, K. C., Williamson, C. H. D., Willis, A. D., Xu, Z. Z., Zaneveld, J. R., Zhang, Y., Zhu, Q., Knight, R., and Caporaso, J. G.: Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2, Nature Biotechnology, 37, 852–857, https://doi.org/10.1038/s41587-019-0209-9, 2019.
Brankovits, D., Pohlman, J. W., Niemann, H., Leigh, M. B., Leewis, M. C., Becker, K. W., Iliffe, T. M., Alvarez, F., Lehmann, M. F., and Phillips, B.: Methane-and dissolved organic carbon-fueled microbial loop supports a tropical subterranean estuary ecosystem, Nat. Commun., 8, https://doi.org/10.1038/s41467-017-01776-x, 2017.
Burd, A. B., Hansell, D. A., Steinberg, D. K., Anderson, T. R., Arístegui, J., Baltar, F., Beaupré, S. R., Buesseler, K. O., DeHairs, F., Jackson, G. A., Kadko, D. C., Koppelmann, R., Lampitt, R. S., Nagata, T., Reinthaler, T., Robinson, C., Robison, B. H., Tamburini, C., and Tanaka, T.: Assessing the apparent imbalance between geochemical and biochemical indicators of meso- and bathypelagic biological activity: What the @$#! is wrong with present calculations of carbon budgets?, Deep Sea Res. 2 Top Stud. Oceanogr., 57, 1557–1571, https://doi.org/10.1016/j.dsr2.2010.02.022, 2010.
Callahan, B. J., McMurdie, P. J., Rosen, M. J., Han, A. W., Johnson, A. J. A., and Holmes, S. P.: DADA2: High-resolution sample inference from Illumina amplicon data, Nat. Methods, 13, 581–583, https://doi.org/10.1038/nmeth.3869, 2016.
Civitarese, G., Gačić, M., Lipizer, M., and Eusebi Borzelli, G. L.: On the impact of the Bimodal Oscillating System (BiOS) on the biogeochemistry and biology of the Adriatic and Ionian Seas (Eastern Mediterranean), Biogeosciences, 7, 3987–3997, https://doi.org/10.5194/bg-7-3987-2010, 2010.
Civitarese, G., Gačić, M., Batistić, M., Bensi, M., Cardin, V., Dulčić, J., Garić, R., and Menna, M.: The BiOS mechanism: History, theory, implications, Progress in Oceanography, https://doi.org/10.1016/j.pocean.2023.103056, 2023.
De Cáceres, M., Cáceres, C., and Legendre, P.: Associations between species and groups of sites: indices and statistical inference, Ecology, 90, 3566–3574, https://doi.org/10.1890/08-1823.1, 2009.
De Corte, D., Yokokawa, T., Varela, M. M., Agogué, H., and Herndl, G. J.: Spatial distribution of Bacteria and Archaea and amoA gene copy numbers throughout the water column of the Eastern Mediterranean Sea, ISME Journal, 3, 147–158, https://doi.org/10.1038/ismej.2008.94, 2009.
del Giorgio, P. A. and Cole, J. J.: Bacterial growth efficiency in natural aquatic systems, Annu. Rev. Ecol. Syst., 29, 503–541, https://doi.org/10.1146/annurev.ecolsys.29.1.503, 1998.
del Giorgio, P. A. and Duarte, C. M.: Respiration in the open ocean, Nature, 420, 379–384, https://doi.org/10.1038/nature01165, 2002.
Dijkhuizen, L. and Harder, W.: Current views on the regulation of autotrophic carbon dioxide fixation via the Calvin cycle in bacteria, Antonie Van Leeuwenhoek, 50, 473–487, 1984.
Ducklow, H. W.: Bacterial Production and Biomass in the Oceans, in: Microbial Ecology of the Oceans, Gasol, J. M. and Kirchman, D. L., Wiley-Liss, New York, 85–119, ISBN 9780471299929, 2000.
Erb, T. J.: Carboxylases in natural and synthetic microbial pathways, Appl. Environ. Microbiol., 77, https://doi.org/10.1128/AEM.05702-11, 2011.
Gasol, J. M., Doval, M. D., Pinhassi, J., Calderón-Paz, J. I., Guixa-Boixareu, N., Vaqué, D., and Pedrós-Alió, C.: Diel variations in bacterial heterotrophic activity and growth in the northwestern Mediterranean Sea, Mar. Ecol. Prog. Ser., 164, 107–124, https://doi.org/10.3354/meps164107, 1998.
Glöckner, F. O., Yilmaz, P., Quast, C., Gerken, J., Beccati, A., Ciuprina, A., Bruns, G., Yarza, P., Peplies, J., Westram, R., and Ludwig, W.: 25 years of serving the community with ribosomal RNA gene reference databases and tools, Journal of Biotechnology, https://doi.org/10.1016/j.jbiotec.2017.06.1198, 2017.
Grossart, H. P., Frindte, K., Dziallas, C., Eckert, W., and Tang, K. W.: Microbial methane production in oxygenated water column of an oligotrophic lake, Proc. Natl. Acad. Sci. USA, 108, 19657–19661, https://doi.org/10.1073/pnas.1110716108, 2011.
Hallam, S. J., Mincer, T. J., Schleper, C., Preston, C. M., Roberts, K., Richardson, P. M., and DeLong, E. F.: Pathways of carbon assimilation and ammonia oxidation suggested by environmental genomic analyses of marine Crenarchaeota, PLoS Biol, 4, 520–536, https://doi.org/10.1371/journal.pbio.0040095, 2006.
Hansman, R. L., Griffin, S., Watson, J. T., Druffel, E. R. M., Ingalls, A. E., Pearson, A., and Aluwihare, L. I.: The radiocarbon signature of microorganisms in the mesopelagic ocean, Proceedings of the National Academy of Sciences, 106, 6513–6518, https://doi.org/10.1073/pnas.0810871106, 2009.
Hazan, O., Silverman, J., Sisma-Ventura, G., Ozer, T., Gertman, I., Shoham-Frider, E., Kress, N., and Rahav, E.: Mesopelagic prokaryotes alter surface phytoplankton production during simulated deep mixing experiments in Eastern Mediterranean Sea waters, Front. Mar. Sci., 5, 1–11, https://doi.org/10.3389/fmars.2018.00001, 2018.
Herndl, G. J. and Reinthaler, T.: Microbial control of the dark end of the biological pump, Nature Geoscience, https://doi.org/10.1038/ngeo1921, 2013.
Herndl, G. J., Reinthaler, T., Teira, E., Van Aken, H., Veth, C., Pernthaler, A., and Pernthaler, J.: Contribution of Archaea to total prokaryotic production in the deep atlantic ocean, Appl. Environ. Microbiol., 71, 2303–2309, https://doi.org/10.1128/AEM.71.5.2303-2309.2005, 2005.
Hügler, M. and Sievert, S. M.: Beyond the Calvin cycle: Autotrophic carbon fixation in the ocean, Ann. Rev. Mar. Sci., 3, 261–289, https://doi.org/10.1146/annurev-marine-120709-142712, 2011.
Jaffe, A. L., Salcedo, R. S. R., and Dekas, A. E.: Abundant and metabolically flexible lineages within the SAR324 and gammaproteobacteria dominate the potential for rubisco-mediated carbon fixation in the dark ocean, bioRxiv [preprint], https://doi.org/10.1101/2024.05.09.593449, 2024.
Jiao, N., Herndl, G. J., Hansell, D. A., Benner, R., Kattner, G., Wilhelm, S. W., Kirchman, D. L., Weinbauer, M. G., Luo, T., Chen, F., and Azam, F.: Microbial production of recalcitrant dissolved organic matter: Long-term carbon storage in the global ocean, Nature Reviews Microbiology, https://doi.org/10.1038/nrmicro2386, 2010.
Karl, D. M., Knauer, G. A., and Martin, J. H.: Downward flux of particulate organic matter in the ocean: a particle decomposition paradox, Nature, 332, 438–441, https://doi.org/10.1038/332438a0, 1988.
La Cono, V., Ruggeri, G., Azzaro, M., Crisafi, F., Decembrini, F., Denaro, R., La Spada, G., Maimone, G., Monticelli, L. S., Smedile, F., Giuliano, L., and Yakimov, M. M.: Contribution of bicarbonate assimilation to carbon pool dynamics in the deep Mediterranean Sea and cultivation of actively nitrifying and CO2-fixing bathypelagic prokaryotic consortia, Front. Microbiol., 9, https://doi.org/10.3389/fmicb.2018.00003, 2018.
Luna, G. M., Bianchelli, S., Decembrini, F., De Domenico, E., Danovaro, R., and Dell'Anno, A.: The dark portion of the Mediterranean Sea is a bioreactor of organic matter cycling, Global Biogeochem. Cycles, 26, https://doi.org/10.1029/2011GB004168, 2012.
Martínez-Pérez, A. M., Álvarez-Salgado, X. A., Arístegui, J., and Nieto-Cid, M.: Deep-ocean dissolved organic matter reactivity along the Mediterranean Sea: Does size matter, Sci. Rep., 7, https://doi.org/10.1038/s41598-017-05941-6, 2017.
Mattes, T. E., Nunn, B. L., Marshall, K. T., Proskurowski, G., Kelley, D. S., Kawka, O. E., Goodlett, D. R., Hansell, D. A., and Morris, R. M.: Sulfur oxidizers dominate carbon fixation at a biogeochemical hot spot in the dark ocean, ISME Journal, 7, 2349–2360, https://doi.org/10.1038/ismej.2013.113, 2013.
McMurdie, P. J. and Holmes, S.: Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data, PLoS One, 8, https://doi.org/10.1371/journal.pone.0061217, 2013.
Mena, C., Reglero, P., Hidalgo, M., Sintes, E., Santiago, R., Martín, M., Moyà, G., and Balbín, R.: Phytoplankton Community Structure Is Driven by Stratification in the Oligotrophic Mediterranean Sea, Front. Microbiol., 10, https://doi.org/10.3389/fmicb.2019.01698, 2019.
Moutin, T. and Raimbault, P.: Primary production, carbon export and nutrients availability in western and eastern Mediterranean Sea in early summer 1996 (MINOS cruise), Journal of Marine Systems, 33, 273–288, 2002.
Nakagawa, T., Mori, K., Kato, C., Takahashi, R., and Tokuyama, T.: Distribution of Cold-Adapted Ammonia-Oxidizing Microorganisms in the Deep-Ocean of the Northeastern Japan Sea, Microbes Environ., 365–372, 2007.
Nielsen, E. S.: The use of radio-active carbon (C14) for measuring organic production in the sea, ICES Journal of Marine Science, 18, 117–140, 1952.
Parada, A. E., Needham, D. M., and Fuhrman, J. A.: Every base matters: Assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples, Environ. Microbiol., 18, 1403–1414, https://doi.org/10.1111/1462-2920.13023, 2016.
Psarra, S., Zohary, T., Krom, M. D., Mantoura, R. F. C., Polychronaki, T., Stambler, N., Tanaka, T., Tselepides, A., and Frede Thingstad, T.: Phytoplankton response to a Lagrangian phosphate addition in the Levantine Sea (Eastern Mediterranean), Deep Sea Res. 2 Top. Stud. Oceanogr., 52, 2944–2960, https://doi.org/10.1016/j.dsr2.2005.08.015, 2005.
R Core Team: R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/ (last access: 3 November 2025), 2020.
Rahav, E., Silverman, J., Raveh, O., Hazan, O., Rubin-Blum, M., Zeri, C., Gogou, A., Kralj, M., Pavlidou, A., and Kress, N.: The deep water of Eastern Mediterranean Sea is a hotspot for bacterial activity, Deep Sea Res. 2 Top. Stud. Oceanogr., 164, 135–143, https://doi.org/10.1016/j.dsr2.2019.03.004, 2019.
Reich, T.: Primal, chemo and bacterial productivity coupled with inorganic nutrient concentrations from an off shore, outgoing transect cruises, PANGAEA [data set], https://doi.org/10.1594/PANGAEA.975231, 2025.
Reich, T., Ben-Ezra, T., Belkin, N., Tsemel, A., Aharonovich, D., Roth-Rosenberg, D., Givati, S., Bialik, M., Herut, B., Berman-Frank, I., Frada, M., Krom, M. D., Lehahn, Y., Rahav, E., and Sher, D.: A year in the life of the Eastern Mediterranean: Monthly dynamics of phytoplankton and bacterioplankton in an ultra-oligotrophic sea, Deep Sea Research Part I: Oceanographic Research Papers, 182, 103720, https://doi.org/10.1016/j.dsr.2022.103720, 2022.
Reich, T., Belkin, N., Sisma-Ventura, G., Berman-Frank, I., and Rahav, E.: Significant dark inorganic carbon fixation in the euphotic zone of an oligotrophic sea, Limnol. Oceanogr., 69, 1129–1142, https://doi.org/10.1002/lno.12560, 2024.
Reinthaler, T., van Aken, H. M., and Herndl, G. J.: Major contribution of autotrophy to microbial carbon cycling in the deep North Atlantic's interior, Deep Sea Res. 2 Top. Stud. Oceanogr., 57, 1572–1580, https://doi.org/10.1016/j.dsr2.2010.02.023, 2010.
Riebesell, U., Kö, A., and Oschlies, A.: Sensitivities of marine carbon fluxes to ocean change, PNAS, https://doi.org/10.1073/pnas.0813291106, 2009.
Robinson, C., Tilstone, G. H., Rees, A. P., Smyth, T. J., Fishwick, J. R., Tarran, G. A., Luz, B., Barkan, E., and David, E.: Comparison of in vitro and in situ plankton production determinations, Aquatic Microbial Ecology, 54, 13–34, https://doi.org/10.3354/ame01250, 2009.
Santinelli, C.: DOC in the Mediterranean Sea, in: Biogeochemistry of marine dissolved organic matter, Elsevier, 579–608, https://doi.org/10.1016/B978-0-12-405940-5.00013-3, 2015.
Santinelli, C., Nannicini, L., and Seritti, A.: DOC dynamics in the meso and bathypelagic layers of the Mediterranean Sea, Deep Sea Res. 2 Top. Stud. Oceanogr., 57, 1446–1459, https://doi.org/10.1016/j.dsr2.2010.02.014, 2010.
Santinelli, C., Hansell, D. A., and Ribera d’Alcalà, M.: Influence of stratification on marine dissolved organic carbon (DOC) dynamics: The Mediterranean Sea case, Prog. Oceanogr., 119, 68–77, https://doi.org/10.1016/J.POCEAN.2013.06.001, 2013.
Schlitzer, R.: Ocean Data View, https://odv.awi.de, last access: 25 February 2025.
Simon, M., Alldredge, A., and Azam, F.: Bacterial carbon dynamics on marine snow, Mar. Ecol. Prog. Ser., 65, 205–211, 1990.
Sisma-Ventura, G., Kress, N., Silverman, J., Gertner, Y., Ozer, T., Biton, E., Lazar, A., Gertman, I., Rahav, E., and Herut, B.: Post-eastern Mediterranean Transient Oxygen Decline in the Deep Waters of the Southeast Mediterranean Sea Supports Weakening of Ventilation Rates, Front. Mar. Sci., 7, https://doi.org/10.3389/fmars.2020.598686, 2021.
Sisma-Ventura, G., Bialik, O. M., Makovsky, Y., Rahav, E., Ozer, T., Kanari, M., Marmen, S., Belkin, N., Guy-Haim, T., Antler, G., Herut, B., and Rubin-Blum, M.: Cold seeps alter the near-bottom biogeochemistry in the ultraoligotrophic Southeastern Mediterranean Sea, Deep Sea Research Part I: Oceanographic Research Papers, 183, 103744, https://doi.org/10.1016/j.dsr.2022.103744, 2022a.
Sisma-Ventura, G., Belkin, N., Rubin-Blum, M., Jacobson, Y., Hauzer, H., Bar-Zeev, E., and Rahav, E.: Discharge of polyphosphonate-based antiscalants via desalination brine: impact on seabed nutrient flux and microbial activity, Environ. Sci. Technol., https://doi.org/10.1021/acs.est.2c04652, 2022b.
Smith, D. and Azam, F.: A simple, economical method for measuring bacterial protein synthesis rates in seawater using, Marine microbial food webs, 6, 107–114, 1992.
Swan, B. K., Martinez-Garcia, M., Preston, C. M., Sczyrba, A., Woyke, T., Lamy, D., Reinthaler, T., Poulton, N. J., Masland, E. D. P., Gomez, M. L., Sieracki, M. E., DeLong, E. F., Herndl, G. J., and Stepanauskas, R.: Potential for chemolithoautotrophy among ubiquitous bacteria lineages in the dark ocean, Science, 333, 1296–1300, https://doi.org/10.1126/science.1203690, 2011.
Tamburini, C., Boutrif, M., Garel, M., Colwell, R. R., and Deming, J. W.: Prokaryotic responses to hydrostatic pressure in the ocean – a review, Environmental Microbiology, https://doi.org/10.1111/1462-2920.12084, 2013.
Techtmann, S. M., Fortney, J. L., Ayers, K. A., Joyner, D. C., Linley, T. D., Pfiffner, S. M., and Hazen, T. C.: The unique chemistry of Eastern Mediterranean water masses selects for distinct microbial communities by depth, PLoS One, 10, https://doi.org/10.1371/journal.pone.0120605, 2015.
Teira, E., Pazó, M., Quevedo, M., Fuentes, M., Niell, F., and Fernández, E.: Rates of dissolved organic carbon production and bacterial activity in the eastern North Atlantic Subtropical Gyre during summer, Mar. Ecol. Prog. Ser., 249, 53–67, https://doi.org/10.3354/meps249053, 2003.
Thingstad, T. F., Krom, M. D., Mantoura, R. F. C., Flaten, G. A. F., Groom, S., Herut, B., Kress, N., Law, C. S., Pasternak, A., and Pitta, P.: Nature of phosphorus limitation in the ultraoligotrophic eastern Mediterranean, Science, 309, 1068–1071, 2005.
Whitman, W. B., Coleman, D. C., and Wiebe, W. J.: Perspective Prokaryotes: The unseen majority, PNAS, https://doi.org/10.1073/pnas.95.12.6578, 6578–6583, 1998.
Yakimov, M. M., Cono, V. La, Smedile, F., Deluca, T. H., Juárez, S., Ciordia, S., Fernández, M., Albar, J. P., Ferrer, M., Golyshin, P. N., and Giuliano, L.: Contribution of crenarchaeal autotrophic ammonia oxidizers to the dark primary production in Tyrrhenian deep waters (Central Mediterranean Sea), ISME Journal, 5, 945–961, https://doi.org/10.1038/ismej.2010.197, 2011.
Zhou, W., Li, Y., Liu, X., He, S., and Huang, J. C.: Comparison of microbial communities in different sulfur-based autotrophic denitrification reactors, Appl. Microbiol. Biotechnol., 101, 447–453, https://doi.org/10.1007/s00253-016-7912-y, 2017.
Zweifel, U., Norrman, B., and Hagström, Å.: Consumption of dissolved organic carbon by marine bacteria and demand for inorganic nutrients, Marine Ecology Progress Series, 101, 23–32, 1993.




