the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
There and back again, a journey of many pathways: conceptualising the marine organic carbon cycle
Maike Iris Esther Scheffold
Inga Hense
Understanding and determining the pathways that organic carbon (OC) takes in the ocean is one of the pressing tasks of our time, as the fate of OC in the ocean is linked to the climate system and the functionality of marine ecosystems. The multitude and complexity of these pathways are typically investigated with sophisticated, mainly quantitative, methods focused on individual pathways in order to resolve their interactions and processes as realistically as possible. In addition to these approaches towards understanding and recreating complexity, there is a need to identify commonalities and differences between individual OC pathways and define their overarching structures. Such structures can provide a framework for the growing number of partly overlapping concepts, which conceptualise selected OC pathways, and promote more systematic comparisons and consistent communication, especially between different disciplines. In response, we propose a (visual) concept in which we define such higher-level “structures” by comparing and condensing marine OC pathways based on their sequences of processes and the layers of the marine system in which they operate. The resulting structures comprise “closed loops”, three remineralisation and two recalcitrant dissolved organic carbon (rDOC) loops that close within marine systems, and “open loops”, condensing pathways leaving the marine system for the atmosphere or deeper sediment layers. In addition, we provide a synthesis of embedded processes, OC pools, and process-performing organisms (agents) embedded in these loops. By translating a definition of the biological carbon pump (BCP) into our concept, we show how the application and discussion of our defined structures facilitate a consistent visualisation, a systematic comparison of differently resolved concepts and studies, and integration of these into the larger picture of the marine OC cycle. As a complement to quantitative studies and descriptions of individual pathways, our concept decomposes the complexity of OC pathways by defining new universal structures. These structures provide a skeleton that can be adapted to different systems and filled with life by the users.
- Article
(3145 KB) - Full-text XML
-
Supplement
(502 KB) - BibTeX
- EndNote
The pathways along which organic carbon (OC) moves through oceanic systems affect not only the climate system (Barange et al., 2017) and ecosystem functioning (Griffiths et al., 2017), but also human well-being and socio-ecological systems (Ullah et al., 2018). Therefore, understanding marine OC pathways and the current and future marine OC dynamics resulting from the multiplicity of these pathways is an essential and very productive focus of ocean research (Jiao et al., 2018). Comprehensive observations and sophisticated numerical models, e.g. by the Joint Global Ocean Flux Study (JGOFS; Doney and Ducklow, 2006), improved carbon budgets (e.g. by Giering et al., 2014), and quantitative estimates of the contribution of individual organisms (e.g. in Bianchi et al., 2021), to name but a few, are continuously expanding our understanding of OC pathways and the marine OC cycle.
Complementing the often-quantitative results, these studies sometimes provide (visual) concepts that abstractly describe and generalise OC pathways as a sequence of processes or a core mechanism. Due to the multitude of disciplines involved, the heterogeneity of ocean systems and the complexity of the marine OC cycle, these concepts often only consider a selection of pathways related to the respective research focus. For example, some studies conceptualise and generalise pathways for specific carbon pools, e.g. dissolved OC in the microbial pump (Jiao et al., 2010; Jiao and Zheng, 2011), for a selection of species such as bacteria in the microbial loop (Azam et al., 1994) or for physical processes of different scales, e.g. large-scale or eddy-subduction export (Levy et al., 2013; Omand et al., 2015).
The different foci and the limited spectrum of the pathways considered lead to concepts that complement each other (focusing on different processes or pools), but also promote partly overlapping sub-concepts. An example is the generalisation of pathways leading to the biota-induced vertical gradient of dissolved inorganic carbon (DIC) in the oceans, described by the concept of the biological carbon pump (BCP). Several sub-concepts of the BCP have emerged, describing, among other things, the transport of carbon into and out of specific water layers, such as the mixed layer pump (Gardner et al., 1995), or carbon export by species-specific behaviour, such as the lipid pump (Jónasdóttir et al., 2015). Recent approaches to further generalise the pump concept by defining its main functions, e.g. particle injection by Boyd et al. (2019), show the need to define structural elements to make concepts such as the BCP more comparable, comprehensive, systematic, and adaptable.
It is plausible and useful that studies on individual OC pathways or systems produce specific and small-scale sub-concepts. However, in science, there is an additional need to identify commonalities and to find and define basic unifying structures (Scheiner and Willig, 2011). So far, no attempt has been made to summarise and generalise OC pathways and conceptual ideas into an overarching general concept that represents structures of the marine OC cycle.
Existing concepts, especially those aiming at a more comprehensive representation of the marine OC cycle, are often not visually congruent within respective graphics or compared to schemata in other publications. Processes and pathways are for instance not represented with the same level of detail. For example, Steinberg and Landry (2017), Cavan et al. (2019), Anderson and Ducklow (2001), and Boscolo-Galazzo et al. (2018) visually detach processes from their products, such as DIC, or do not mention some products in the figures at all. Although the aim of such studies is not to create congruent conceptual representations of the marine OC cycle, their visualisations are still useful tools to highlight their research focus in an overarching picture. However, we would like to emphasise that graphics are a visualisation of the mapper's mental concepts. By deciding what to visualise and at what resolution, and by omitting information, parts of these mental concepts are obscured, which can make it difficult to understand and use the concepts for studies other than the one for which it was created. Graphics are powerful tools for disseminating information, displaying concepts, and promoting discussion (Margoluis et al., 2009). Incongruent graphics do not exploit that full potential.
The lack of an overarching (and congruently visualised) concept of the marine OC cycle can reduce the transparency of the scientific process and can make comparisons and discussions as well as the adaptation of already existing concepts and ideas more difficult (Scheiner and Willig, 2011). Different resolutions and definitions of pathways and overarching structures risk misunderstanding and miscommunication in education (Fortuin et al., 2011), among young but also more experienced researchers or in interdisciplinary communities (Heemskerk et al., 2003) and may foster a growing number of sub-concepts (Scheiner and Willig, 2011), some of which may overlap.
To reduce this risk, we propose stepping back from quantitative, specific, and numerically advanced research and summarising and generalising what is known about the marine OC cycle and pathways. The result of this step is a general concept that does not represent specific carbon processes or a single pathway but defines common structures of all pathways. We define these structures in linguistic and visual units by comparing and condensing similarities of possible OC pathways in the marine system. The result is the definition of several structures of “closed” and “open” OC loops that include all pathways that close within the marine system or leave the system for the deeper sediment or atmosphere.
The resulting concept facilitates (1) comparing models and conceptual frameworks, (2) synthesising definitions and scientific language, (3) adding new scientific knowledge in a congruent and structured way, (4) identifying research gaps and inconsistencies, and (5) placing finite pathways into an overarching framework of the marine OC cycle. In this way, the concept can help researchers from different disciplines to facilitate research design, discuss individual concepts, and improve interdisciplinary communication, collaboration, and scientific education.
In the following, we describe how we develop our concept based on the questions. (1) What are the different pathways for an OC compound in marine systems? (2) Which structures can be condensed? (3) Which processes, pools, and agents are embedded in these structures? By answering the first two questions, we obtain a concept of universal structures of marine OC pathways. By answering question (3), we identify the processes, pools, and agents embedded in these structures, which allow for the definition of smaller-scale structures that can be adapted to specific research questions and marine systems. In the discussion, we describe as an application example how a definition of BCP can be translated into our concept, and discuss the add-ons of this representation.
Given that we conceptualise only the OC pathways (for a definition of relevant terms of the concept, see Table 1), we do not resolve carbonate and alkalinity interactions, and do not display marine carbonate systems within our concept.
In addition, we focus on the OC that remains within the marine system, i.e. the water column as well as the upper sediment that still interacts with the water column. Therefore, we only consider pathways that start as OC within the surface water, acknowledging that this initial position (Table 1) is an artificial construct since cycles do not start (or end) anywhere, and marine carbon may originate from terrestrial run-off, atmospheric deposition, or photosynthesis. In this concept, we do not describe in detail those OC pathways that have left the marine system, either for the atmosphere or for deeper sediment layers that do not interact with the water column, and we assign them to open loops. These loops close too, but outside our focal marine system.
It is irrelevant for our concept how much time an organic compound spends in the pathway. As such we are not interested in resolving the timescales of pathways and the accumulation of standing stocks of OC in the system. Thus, it is the same pathway when OC remains in the standing stock of a whale throughout its life and is respired at the surface right before its death and when OC is respired by a whale at the water surface immediately after being consumed. However, we do implicitly include timescales of pathways, since we consider different spatial scales closely connected to temporal scales (Dickey, 1990).
We provide a qualitative concept and are not interested in the amount of carbon that passes through the different pathways or the probabilities of OC to do so. We consider all pathways as being equally possible by assuming that each carbon compound finds the conditions for each pathway at the same time. For instance, the system provides suitable consumers that reduce the sinking of material and at the same time gives a spatio-temporal mismatch with consumers that favours sinking.
To identify structures of higher resolution (Sect. 3.2), we operationally subdivide OC into different pools if the pathways involve OC of different size, volatility, and lability. In such cases, we distinguish particulate organic carbon (POC), embedding living and non-living OC with sizes larger 0.2 µm (Kharbush et al., 2020), aggregates, and marine snow; dissolved organic carbon (DOC), defined as non-living carbon smaller 0.2 µm (Kharbush et al., 2020); and volatile organic compounds (VOCs), such as dimethyl sulfate and CH4. In addition, we separately consider recalcitrant (or refractory) DOC (rDOC), defined here as DOC that is remineralised on timescales between 1.5 and 40 000 years for semi-labile to ultra-refractory DOC (Hansell, 2013), as opposed to 0.001 years for labile DOC (Hansell, 2013). We consider rDOC separately from DOC because rDOC is considered the only form of OC that accumulates in the water column in quantities relevant to the climate system (Jiao et al., 2010; Jiao and Zheng, 2011). We also include DIC as an intermediate pool. While this DIC pool consists of various IC molecules, we do not differentiate among them within our concept.
3.1 Main structures of the marine organic carbon cycle
Our concept is based on the comparison and condensation of possible OC pathways using state-of-the-art knowledge. To this end, we generate a literature-based pathway concept (see Supplement S1) by collecting and mapping the different pathways that an OC compound can “take” within the marine OC cycle based on a non-systematic literature review. The individual pathways in this concept are defined by sequences of processes (Table 1), such as sinking and remineralisation, and either return to the initial position in the surface water or leave the marine system for the sediment or the atmosphere. We compare the OC pathways in the literature-based pathway concept and condense their similarities into generally applicable structures.
The structures, e.g. closed loops, are stripped of any processes, pools, or involved agents (definitions see Table 1). We add this information in the next step (Sect. 3.2) allowing for the definition of additional structures of higher resolution.
To explain how the pathways of the literature-based pathway concept can be compared and condensed to define structures of the marine OC cycle, we use as an analogy a town with a sandbank separated by a lagoon. The inhabitants of the town regularly visit the sandbank to spend their evenings at the beach. A route planner, comparable to our literature-based pathway concept, shows 100 individual pathways that end at the beach. These pathways are similar, but all differ in the overall sequence of streets and vehicles used.
There is, however, a common denominator for all pathways. To reach the beach, the lagoon must be crossed. This condition is independent of the way of crossing. People reach the sandbank in different ways, e.g. by public ferry or private boat. The result “people reach the sandbank” and the general functionality “crossing the lagoon” of these processes coincide. Therefore, we define “crossing the lagoon” as a functional segment (summarised function of the involved processes with the same general functionality, Table 1) common to all pathways to the beach. It should be noted that this does not mean that all pathways ONLY need this functional segment. The functional segment “crossing the lagoon” is at least required to reach the beach and the bottleneck of ALL beach pathways.
At a higher resolution, which allows more complexity, differences of the beach pathways can be identified and grouped by defining the functional segments shared by these groups. For instance, people who do not live at the harbour front (functionality: “living behind the harbour front”) use one of three roads to reach the harbour (functionality: “reaching the harbour”). These pathways share the sequence of the functional segments “living behind the harbour front”, “reaching the harbour” and “crossing the lagoon”. People living at the harbour front (functionality: “living at the harbour front”) only have to cross the lagoon and share the sequence of “living at the harbour front” and “crossing the lagoon”.
Based on these distinct sequences of functional segments, different structures can be defined. The most general and superordinate structure is the “entire town–beach” structure defined by the functional segment “crossing the lagoon”, which is common to all pathways. That sequence is the minimum sequence shared by all pathways and defines the highest-level structure. At a higher resolution, a distinction can be made between a “harbour front–beach” structure (living at the harbour front and crossing the lagoon) and a “behind the harbour–beach” structure (living behind the harbour front, reaching the harbour and crossing the lagoon). The sequences of functional segments minimally describe all pathways within these structures.
The resolution and thus the definition of structures is a matter of choice. One could for example also distinguish other structures based on the method of crossing the lagoon or find further differences and commonalities between the pathways in the rest of the town and define additional structures. However, assuming that the rest of the town has a very diverse and complicated road network, the “harbour front–beach” and the “behind the harbour–beach” structure may be sufficient to define, for instance, pressure points and bottlenecks when construction works block the three streets to the harbour.
Similarly to the description above, we define structures of the marine OC pathways based on the literature-based pathway concept (see Supplement S2 for a schematic of the methodological steps). One structure that immediately catches the eye are pathways that loop inside or outside the marine system. We define these structures as closed and open loops. The closed loops are the highest structure in the marine OC cycle and the focus of this study. In the following, we define general structures hierarchically below the closed loops by comparing the pathways of these loops as described above. The structures we want to define should be as general as possible while still covering relevant differences.
We identify six functional segments that are necessary to describe the desired structures (Fig. 1): OC position change (A), formation of rDOC (B), rDOC conversion to DOC (C), OC remineralisation (D), DIC upward position change (E), and DIC uptake by primary producers (F). We recognise that excluding the rDOC-related functional segments would further reduce the number of functional segments and structures. However, as described earlier, rDOC is relevant to the climate system and is related to very different phenomena and processes. So although it may not technically be the minimum solution, it is the minimum solution that still captures relevant differences.
The depths OC reaches on its pathways is another relevant difference that we want to resolve, as these depths affect the function of OC in the ecosystem (e.g. as a food source for benthic organisms), the environmental conditions it encounters (e.g. bioturbation), and the time it takes to return to the surface layer (e.g. years or decades). However, the functional segment OC position change (A) does not provide information on whether the position change ends in the water column or in the sediment.
Hence, to unambiguously define structures that account for the differences described above we need to add spatial information. To systematically add this information, we define five spaces, volumes with distinctly different environmental conditions and processes. After general considerations of the ocean layers, the surface layer space (SLS) encounters sufficient light to support photosynthesising organisms and primary production. Seasonal and continuous mixing counteract material loss and keep matter close to remineralisers. In the water column space (WCS) below the well-mixed layer, mixing occurs less frequently, more slowly or very infrequently, depending among other things on the water depth (DeVries et al., 2012). Matter takes more time to resurface and may escape remineralisers due to changing positions or its recalcitrant or degraded character (Baker et al., 2017). In the upper sediment space (USS), remineralisers also remineralise highly degraded material as it remains in their vicinity longer than in the water column (Middelburg, 2019). The lower sediment space (LSS) is largely abiotic and undisturbed and allows lithification processes. In addition, we define the atmosphere space (AS) above the marine system. The use and choice of spaces depend on the intended resolution of the structures. Users of the concept can change the spaces, e.g. by subdividing the WCS, resulting in a different number of closed loops, or omit the spatial extent completely if they aim for an even more general description than ours. However, if the minimum number of closed loops is to be conceptually described at the same level of resolution as ours, each coastal system must be represented by at least two spaces (SLS and USS) and pelagic marine systems by at least three spaces (SLS, WCS, and USS). In the following we represent functional segments with the corresponding letters; the text in the following square brackets refers to the spaces in which the associated processes end or take place (syntax example: A [WCS], OC position change ending in the WCS).
Based on the unique combinations of (1) the sequence of functional segments and (2) the involved spaces, we now define three closed remineralisation and two rDOC loops (Fig. 1 and Table 2).
The remineralisation loops comprise a surface layer remineralisation loop (SLRL), a water column remineralisation loop (WCRL), and an upper sediment remineralisation loop (USRL; Table 2). All three loops include pathways through which OC is remineralised to DIC (D), which is taken up by primary producers in the SLS (F [SLS]). The functional segments OC position change (A) and DIC upward position change (E), as well as the space in which the OC is remineralised, distinguish the remineralisation loops. The WCRL includes pathways that lead to a downward position change of OC into the WCS, remineralisation in the WCS, and an upward position change of DIC into the SLS, where it is taken up (WCRL: A [WCS] → D [WCS] → E [SLS] → F [SLS]). An exemplary WCRL pathway involves OC uptake by zooplankton in the SLS, its migration into and respiration in the WCS, and the upward mixing of the resulting DIC into the SLS where it is taken up by primary producers (WCRL: A [WCS] → D [WCS] → E [SLS] → F [SLS]). If zooplankton respiration occurs in the SLS, the pathway belongs to the SLRL (SLRL: D [SLS] → F [SLS]). We define the USRL analogous to the WCRL, but with remineralisation taking place in the USS (USRL: A [USS] → D [USS] → E [SLS] → F [SLS]).
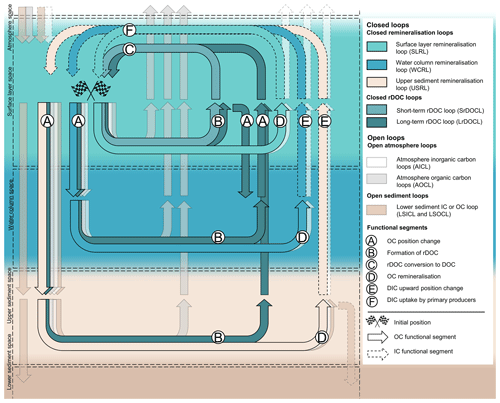
Figure 1General structures of the marine OC cycling with three closed remineralisation and two closed rDOC loops, the spaces and the involved functional segments. Open loops are only displayed with transparent colours as they are not our focus.
The two functional segments formation of rDOC (B) and rDOC conversion to DOC (C) in the SLS are part of another set of closed loops, the rDOC loops (Fig. 1 and Table 2). The rDOC loops describe the change of labile OC to more recalcitrant forms, its persistence in the system, and its return to bioavailable forms in the SLS. We differentiate between a short-term rDOC loop (SrDOCL), rDOC that accumulates in the surface water on timescales of human life, and a long-term rDOC loop (LrDOCL), rDOC that can persist in the entire water column on geological timescales. The SrDOCL is defined by the formation of rDOC (B) and rDOC conversion to DOC (C) in the SLS (SrDOCL: B [SLS] → C [SLS]), while the LrDOCL additionally comprises the functional segment OC position change (A), with accumulation mostly or even entirely in the WCS (LrDOCL: B [SLS] → A [WCS/USS] → A [SLS] → C [SLS] or A [WCS/USS] → B [WCS/USS] → A [SLS] → C [SLS]). In contrast to the remineralisation loops, we do not explicitly consider an rDOC loop in the upper sediment, as the temporal scales of rDOC produced there or in the water column overlap to our knowledge. Therefore, the LrDOCL includes rDOC production in the USS alongside its transport to the WCS. It has to be noted that only rDOC that reaches the surface and is converted back into more bioavailable forms in the SLS belongs to the LrDOCL (LrDOCL: A [SLS] → C [SLS]). rDOC can for instance be part of the WCRL when remineralised in WCS (WCRL: B [SLS] → A [WCS] → D [WCS] → E [SLS] → F [SLS]). Because of the climatic importance of rDOC, we distinguish rDOC from DOC as described before. Technically, however, rDOC represents an intermediate “storage” step of remineralisation or open loops.
All loops comprise a continuum of processes that are not addressed in the defined sequences of functional segments. For example, the SLRL also includes pathways on which OC is transported and processed below the SLS but returns to the SLS as OC to be remineralised and used by primary producers (SLRL: A [WCS] → A [SLS] → D [SLS] → F [SLS]). To avoid double counting when assigning pathways like this to one of our defined loops, two separation rules apply. The first rule states that the space of the ultimate remineralisation before entry and reuse in the SLS defines the remineralisation loop. OC that is remineralised several times in different spaces is part of the SLRL if it is eventually remineralised in the SLS before being taken up by primary producers in the SLS. Similarly, OC belongs to the WCRL or USRL if it is ultimately remineralised in the WCS or USS. The second rule states that rDOC leaving the surface or produced below the SLS always belongs to the LrDOCL (Table 2).
For the minimal description of the remineralisation and rDOC loops, the sequences of the above-defined functional segments are sufficient and unambiguous. However, users of the concept can identify and combine other functional segments to define different higher-resolution structures.
Table 2Summary of sequences of functional segments and spaces defining the remineralisation and rDOC loops. The separation rule comes into play, when assigning a pathway to one of the defined loops. Spaces in square brackets indicate the spaces where the processes happen or end. Spaces in bold text are the naming spaces of this loop. Spaces in non-bold font are intermediate or “walk-through” spaces. Loops: surface layer remineralisation loop (SLRL), water column remineralisation loop (WCRL), upper sediment remineralisation loop (USRL), and short- and long-term rDOC loops (SrDOCL, LrDOCL). Spaces: surface layer space (SLS), water column space (WCS), and upper sediment space (USS). Functional segments: OC position change (A), formation of rDOC (B), rDOC conversion to DOC (C), OC remineralisation (D), DIC upward position change (E), and DIC uptake by primary producers (F).

Although we focus on the closed loops, it is noteworthy that there are parallel open loops of carbon that close outside the marine systems, e.g. in the atmosphere. We define four structures of open loops. Atmospheric IC loops (AICLs) describe the outgassing of DIC, produced in different spaces, to the atmosphere. Atmospheric OC loops (AOCLs) comprise the exit of marine OC, marine aerosols, VOCs, and CH4 through the surface to the AS, e.g. via fish predation by birds or outgassing. Lower sediment IC loop (LSICL) and lower sediment OC loop (LSOCL) describe the burial and lithification of carbon in the LSS, entering geological cycling.
3.2 Embedded processes, pools, and agents
Having defined the structures of remineralisation and rDOC loops, we now add and describe global processes, pools, and agents embedded in each functional segment (Fig. 2 and Table ). This addition allows us to define structures with higher resolution and to link and complement our concept with existing ones. In this context, global means that the process mechanisms are globally valid, but that the frequency, extent, initialisation, and triggers of these processes differ. We focus on non-anthropogenic processes and the previously defined functional segments. This means that, for example, upward position changes of POC or DOC are not resolved.
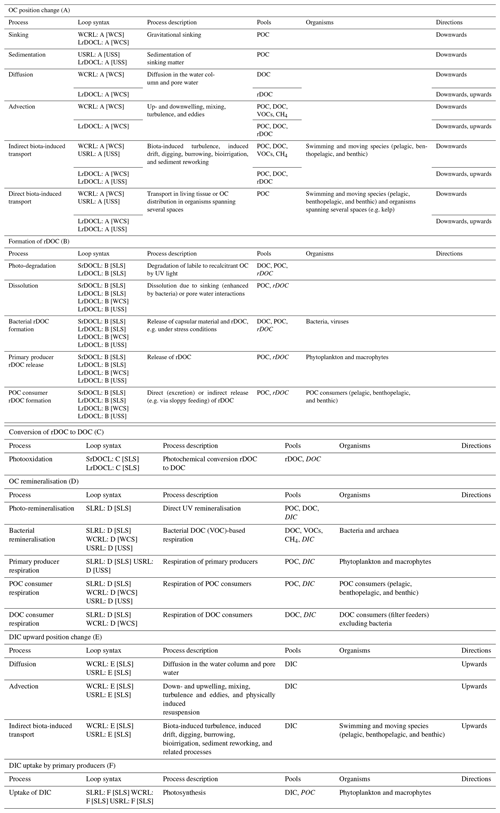
Two of the three remineralisation loops include the functional segments OC position change (A) and DIC upward position change (E). Processes belonging to functional segments A and E include sinking, diffusion and advection, and direct and indirect biota-induced transport.
Organic compounds that sink from one space in the water column to another are usually either large or dense, or escape consumption or dissolution in the upper space (De La Rocha, 2006). Sedimentation and compaction by subsequent matter are the analogous processes within the sediment–water interface and sediment. Matter is compacted by the weight deposited over it and sinks as it loses volume. Sinking and sedimentation always act downwards and are confined to POC. Gravity-induced sinking (and sedimentation) is thus part of any functional segment A of POC (Fig. 2).
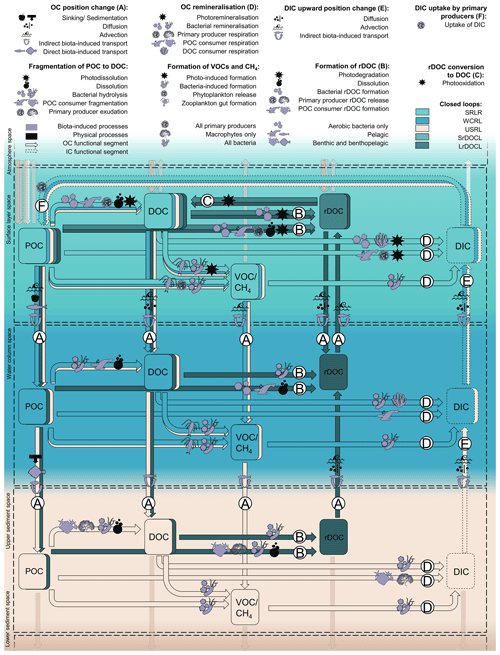
Figure 2Defined OC structures with functional segments A–F, spaces and embedded processes, OC pools, and involved organisms. Open loops are indicated by transparent colours. Organisms can be agents (producing DOC by sloppy feeding) and part of the carbon pool (consumers as part of POC respiring DIC) at the same time. Fragmentation processes and pathways for DOC and VOCs/CH4 are included. As they are not resolved in the loops, these pathways are not marked with capital letters.
(r)DOC (DOC and rDOC) and DIC potentially diffuse in all directions, following large- or small-scale gradients in the water column, at the sediment–water interface and in the pore water of sediment. We assume that (r)DOC concentrations decrease with depth (Hansell, 2013) but are higher in the sediment than in the overlying water (Burdige et al., 1999; Rowe and Deming, 2011) and that DIC concentrations increase with depth (Oka, 2020). Following these gradients, (r)DOC diffuses downwards in the water column and upwards in and out of the sediment (A of (r)DOC in Fig. 2) and DIC always diffuses upwards (E). The upward diffusion of non-refractory DOC from the sediment is not considered in the defined functional segments as upward movements are not common to all pathways of the remineralisation loops.
Other physically induced position changes are related to water or sediment mass movements based on advection. These include large-scale upwelling and downwelling water movements, seasonal mixing, wind-induced turbulence and eddies, and storm-induced resuspension. Advection is globally applicable although its direction, magnitude, and frequency vary. The advection-induced position change occurs in the functional segments A and E. Advection does not act downwards into the sediment but upwards in the form of resuspension. Resuspension is only included for rDOC and is limited to the upper part of the sediment, as physical perturbation does not commonly reach below 10 cm (Boudreau, 1998; Bunke et al., 2019).
Biota-induced transport involves the direct transport of OC in the living tissue of migrating organisms (e.g. a fish feeds in the SLS, migrates down, and dies in the WCS) as well as the internal flux of OC in organisms that span different spaces (e.g. macrophytes living in the SLS and the USS; Middelburg, 2019). Organisms change their position in the water column (e.g. via diel vertical migration; Steinberg et al., 2002) or in the sediment (e.g. via burrowing; Middelburg, 2019) and produce faecal pellets or die after the position change. The result of direct biota-induced position change is POC of all sizes, e.g. living organisms and roots, faeces, and carcasses. Direct biota-induced position change works in all directions and is involved in the functional segment A of POC.
Indirect biota-induced transport comprises biogenic turbulence (Kunze et al., 2006; Huntley and Zhou, 2004), as well as induced drift, which describes the transport of substances that adhere to the bodies of swimming organisms (Katija and Dabiri, 2009). Indirect biota-induced position change in the sediment is related to, among others, bioturbation (Berke, 2010), associated with sediment reworking, and resuspension, and bioirrigation (Kristensen et al., 2012), which leads to inflows of ocean water into the sediment. Indirect biota-induced position change works in all directions and is involved in the functional segment of A for (r)DOC and POC and E in the water column and the sediment.
The following processes belong to the functional segment OC remineralisation (D). We define remineralisation as the provision of DIC based on OC and restrict it to the spaces above the LSS, assuming that remineralisation in the LSS is negligible.
Light-induced photo-remineralisation, the only physically induced remineralisation, directly oxidises DOC and POC to IC (Mopper and Kieber, 2002; Mayer et al., 2009) and works only in the SLS. We include this process in D in the SLS.
Bacteria and archaea remineralise DOC in functional segment D in every space above LSS, also under different oxygen conditions. The DOC is either of allochthonous origin (e.g. entering via riverine input; Dai et al., 2012), or of autochthonous origin based on living or non-living POC. For instance, POC dissolves while sinking (Carlson and Hansell, 2015), is fragmented by turbulence (Ruiz, 1997; Briggs et al., 2020), or photo-dissolved (Mayer et al., 2006). Consumers reduce the size of organic POC by sloppy feeding on living and non-living POC, e.g. by zooplankton coprorhexy (Lampitt et al., 1990), by producing small metabolites, excreting DOC (Lampert, 1978), or by swimming or moving (Dilling and Alldredge, 2000; Goldthwait et al., 2004). Further, primary producers exudate DOC in the water column (e.g. under nutrient-limited conditions or viral lysis; Azam and Malfatti, 2007) and in the sediment (by macrophytes; Duarte and Cebrián, 1996). Bacteria, for their part, hydrolyse POC to DOC (Smith et al., 1992) and additionally release DOC by viral lysis (Middelboe et al., 1996).
The transformation from POC to DOC (arrows from POC to DOC, Fig. 2) that takes place before bacterial remineralisation is not defined as a functional segment of the remineralisation loops, as not every OC compound needs to undergo one of these changes to be remineralised. However, when considering only DOC-based pathways, for example, the change in OC size from POC to DOC can be defined as a common functional segment and used to define structures such as POC-DOC remineralisation loops.
In addition, bacteria can oxidise VOCs and CH4 (D of VOCs CH4 in Fig. 1), as shown, for example, by Halsey et al. (2017). The VOCs and CH4 originate from abiotic processes such as photochemical degradation of DOC (Kieber et al., 1989) and biogenic processes, e.g. production by phytoplankton (Lenhart et al., 2016) and zooplankton in anaerobic areas of their guts (Weber et al., 2019; Schmale et al., 2018).
Another form of remineralisation is respiration by living organisms other than bacteria. Primary producers respire in the photic SLS. The roots of macrophytes additionally produce DIC in the USS at night (Pedersen et al., 1995). Higher trophic levels, POC consumers (e.g. zooplankton and fish), and non-bacterial DOC consumers (e.g. suspension-feeding sponges at the sediment–water interface; Wooster et al., 2019), also remineralise by respiration. Therefore, we include remineralisation by primary producers in functional segment D in the SLS and USS, respiration by DOC consumers in the SLS and WCS, and respiration by POC consumers in all spaces with aerobic conditions above the LSS.
Once OC is remineralised to DIC, this DIC is transported by the above-described processes of position change to the SLS (E [SLS]). Subsequently, primary producers take up the DIC for photosynthesis (F [SLS]) and close the remineralisation loops.
The rDOC loops include the formation of rDOC (B), the reconversion to DOC in the SLS (C), and, in the case of the LrDOCL, the OC position change (A). We present some of the involved abiotic and biotic processes that have been reviewed elsewhere, e.g. in Legendre et al. (2015).
Table 3Processes embedded in the functional segments of the defined loops. Pools noted in italics are products of the processes. Processes end or take place in the spaces in square brackets in the loop syntax.
UV light can change the lability and increase recalcitrant components of the DOC pool via photodegradation (Benner and Biddanda, 1998; Hansell, 2013) (B [SLS]). Biota supply rDOC via successive microbial processing of DOC (Jiao et al., 2010; Jiao and Zheng, 2011), the release of capsular material by bacteria (Stoderegger and Herndl, 1998), bacterial hydrolysis of POC (Jiao and Zheng, 2011), bacterial stress responses to low-labile DOC and unfavourable nutrient conditions (Stoderegger and Herndl, 1998), and successive consumption by higher trophic levels (Jiao and Zheng, 2011). In addition, some phytoplankton directly exudates rDOC (Jiao and Zheng, 2011). Both microbes and phytoplankton also release rDOC due to viral lysis of host cells (Jiao and Zheng, 2011). Furthermore, processes that convert living and non-living POC into DOC, e.g. dissolution, can dilute DOC (Fig. 2, arrow from POC to rDOC) to the point where it can no longer serve as sufficient nutrition for microbes and can be considered technically recalcitrant (Arrieta et al., 2015).
rDOC that stays in or returns to the SLS, via the position change processes described above (A [SLS]), can be converted back to more bioavailable forms by photooxidation (C [SLS]; Kieber et al., 1989). We consider pathways with other rDOC removal processes, such as direct light-induced oxidation from rDOC to DIC (Shen and Benner, 2018), sorption of rDOC on POC (Hansell et al., 2009), and hydrothermal removal mechanisms in hydrothermal vents or the Earth's crust (Lang et al., 2006), as parts of closed remineralisation or open loops. Once the rDOC is converted to DOC in the SLS, the rDOC loops are closed.
Based on these embedded processes, pools, and agents, we can now define structures of higher resolution. For example, for SLRL, six structures can be defined based on the carbon pools involved: POC-SLRL, POC-DOC-SLRL, POC-DOC-VOC CH4-SLRL, POC-VOC CH4-SLRL, DOC-SLRL, and DOC-VOC CH4-SLRL. Depending on the research question or desired level of detail, multiple structures can be defined based on the processes and agents involved. The higher the resolution of the structure, the more the structures resemble descriptions of individual pathways. In the following discussion, we use the example of the BCP to show what different structures can look like and what insights a comparison of such different structures can provide.
Our concept of the marine OC cycle condenses pathways into superordinate structures and provides an overview of embedded processes, pools, and agents, which allows us to resolve structures of smaller scale and higher resolution. Our overarching structures complement existing concepts of OC pathways and processes in the ocean, providing a basis for using a consistent terminology. As such, the concept facilitates comparing different definitions of conceptualised pathways, integrating new findings, and placing pathways of finite-length scale in a broader framework.
To discuss some of these aspects in an application example, we translate pathways of the BCP into our concept (Fig. 3). Based on Giering and Humphreys (2020)1, who define the BCP as “the collection of marine biogeochemical processes that convert dissolved inorganic matter in the surface ocean into biomass and transport this to the ocean interior, where the biomass is returned to its original dissolved inorganic forms”, we illustrate different structures with different resolutions and choices of pathways.
Using the syntax of our concept and functional segments A–F, the defined BCP involves the uptake of IC into biomass in the surface water (F [SLS]) and the OC position change to the interior of the ocean (A [ocean interior]), where it is remineralised to DIC (D [ocean interior]). For simplicity, we disregard rDOC, VOCs, and CH4 and start again with the previously introduced initial position. As it is not clarified in the definition, we assume that the ocean interior does not contain the USS and define it as WCS. Based on this restriction of the ocean interior, we classify the BCP as part of the WCRL or the corresponding AICL (Fig. 3a). Note that we need to add functional segment E to count the BCP with the WCRL as E is not included in the defined BCP.
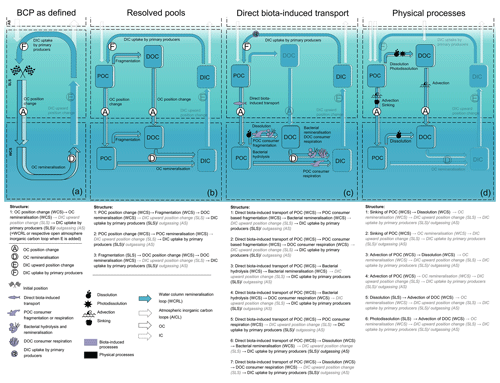
Figure 3The illustration of the BCP defined by Giering and Humphreys (2020) shown with our concept. Panel (a) shows the superordinate BCP structures based on the definition. Panel (b) additionally resolves involved pools. Panel (c) and (d) resolve choices of processes: (c) pathways with direct biota-induced transport and (d) pathways with only physical processes. Functional segments that are transparent, grey, or written in italic font are not explicitly included in the definition of the BCP or the selected pathways. Note that all structures are part of the WCRL or AICL when adding functional segment E to (a)–(c) and D, E, and F to (d). Note further that panel (d) only belongs to the defined BCP if functional segments D and F are added.
If we now resolve the OC pools involved in the BCP (here POC and DOC), we can define three BCP structures of higher resolution (Fig. 3b). Each of these structures defines a part of the BCP. All together, they capture all pathways of the defined BCP. Taking only the pathways with a specific set of processes into account produces structures that no longer comprise all pathways of the BCP. For example, focusing on pathways with direct biota-induced transport (A) results in seven structures. These structures only serve structure 2 from Fig. 3b and thus represent only a part of the defined BCP. This part resembles other concepts of BCPs such as the mesopelagic migrant pump and the seasonal lipid pump (Boyd et al., 2019). Focusing on the purely physically induced pathways of the BCP leads to six different structures that do not resolve remineralisation (D) and DIC uptake (F) as they are non-physical processes (Fig. 3d). These six structures could potentially serve all of the higher-level structures in Fig. 3b of the defined BCP if we add D and F, but they also do not cover all of the pathways of the BCP (see Fig. 3c). Nevertheless, the structures in Fig. 3(d) resemble some, often more traditional, concepts of the BCP, e.g. from Hansell and Carlson (2001) and De La Rocha (2006), which do not explicitly consider DIC uptake and remineralisation.
Integrating the BCP definition of Giering and Humphreys (2020) into our concept illustrates where the BCP concept shows ambiguity and may need to be refined to concretise which pathways belong to the BCP and which do not. Giering and Humphreys (2020) give “ocean interior” as the spatial constraints of the pathways of the BCP. We translate ocean interior as WCS, as no further depth constraints are given. Other BCP definitions constrain depth more concretely, for example describing the BCP as export (Buchan et al., 2014; Hansell and Carlson, 2001) and sequestration fluxes (Sigman and Haug, 2004) acting at depths below 100 and 1000 m (Passow and Carlson, 2012). This ambiguity of the space in which the BCP operates means that we may identify pathways as being part of the BCP that are in fact not, while excluding others that actually are. It is therefore necessary to define the spaces in which the BCP operates more distinctly. Subdividing the WCS into several spaces, e.g. a space below a sequestration depth, may thus be more appropriate for the representation of the BCP, as the definition of spaces allows for refinement of the pathways that belong to a structure.
A similar vagueness as seen in the spaces applies to the OC pools involved. Does the BCP include pathways based on DOC in the SLS (e.g. as defined by Honjo et al., 2014) or not (as defined by De La Rocha, 2006)? DOC is a relevant carbon flux to the deep sea, especially in oligotrophic areas (Roshan and DeVries, 2017). Therefore, and because the definition of Giering and Humphreys (2020) does not explicitly exclude the DOC pool, we resolve DOC in our illustration of the BCP in Fig. 3b. However, the presentation would also work without DOC. In such a case, our concept shows which pathways are missing by dispensing DOC.
Illustrating what is missing also allows placing individual pathways and concepts such as the BCP into a broader framework. For example, mentioning pathway section E is essential in order to place the BCP in the OC cycle, as there is no dead end in nature. Furthermore, our approach helps to identify how different sub-concepts fit into more general definitions (Fig. 3b–c compared to Fig. 3a), but also where some inconsistencies might occur, e.g. remineralisation included in Fig. 3a–c and not Fig. 3d. In addition, it facilitates identifying which pathways are not resolved and the potential informative value of studies based on a limited number of pathways. In Fig. 3c, for example, the DOC in the WCS comes only from fragmentation of POC. If fragmentation processes decrease significantly, this does not necessarily mean a decrease in remineralisation of DOC (D [WCS]), as DOC can also originate from the SLS. A study based on the pathways of Fig. 3c will not consider DOC from the SLS and therefore has limited informative value about changes in the remineralisation of DOC. All mentioned considerations are already part of most studies and publications. But we provide a new tool to systematise these considerations and make them more comparable.
The BCP example further shows how new concepts and processes can be integrated into our concept. Figure 3d resembles more traditional definitions of the BCP, which focus mainly on physically driven processes. The role of organisms, particularly higher trophic levels, was considered quantitatively secondary and was therefore neglected. Now, however, the contribution of this biota is recognised as relevant to the carbon cycle. For example, large migratory species are linked with nutrient distribution and overall mixing (Roman and McCarthy, 2010), zooplankton have a significant influence on carbon export (Steinberg and Landry, 2017), reptile falls provide an alternative carbon pathway to the sediment (McClain et al., 2019), and fish and mammals contribute to the marine OC cycle through various processes (Martin et al., 2021). With these processes, many new structures emerge, some of which we resolve in Figure 3c. Our concept provides overarching structures that users can bring to life to integrate new insights. Processes, organisms, pathways, and loops can easily be added, changed, or deleted to incorporate new findings or specific systems into general structures.
By generalising structures and providing a congruent visual representation, our concept may reduce potential misunderstanding of the marine OC cycle unintentionally caused by visual concepts of finite-length scale. An example of such a potential misunderstanding is the representation of pathways transporting DIC to depths without resolving what happens to the DIC subsequently, as found in some earlier visual OC concepts (as discussed in the introduction to Boscolo-Galazzo et al., 2018). While these representations are justified for finite-length scale studies, this visual decoupling can lead to the false impression that increased transport of OC to the deep ocean always leads to increased sequestration and storage of atmospheric carbon. However, increased OC export is not necessarily accompanied by increased carbon storage, which depends, among other things, on the ratio of regenerated to pre-formed nutrients and on the carbon that escapes from the deep ocean (Gnanadesikan and Marinov, 2008). The export of carbon to the deep sea is part of carbon processing, but not the whole story, as we can also see from the example of the definition of the BCP. We propose using a concept like ours as a reference in order to address the increasingly interdisciplinary scientific community, to strengthen the coherence of (visual) concepts, and to identify the overarching structures of individual pathways.
The provision of overarching structures comes at the cost of not capturing the complexity of the marine OC cycle. Each OC compound travels its pathway through the OC cycle. An OC compound in the surface ocean may end up on the surface or in the deep sea, decompose, or become recalcitrant, to name just a few possibilities. Each pathway is unique in its sequence of processes. So, there is a multitude of possible pathways. An all-encompassing description of these possibilities is, therefore, neither possible nor meaningful. Accordingly, our concept does not want to and cannot resolve individual pathways. On the contrary, it focuses on overarching structures and the minimal functional segments necessary to describe them. Hence, our concept reduces many pathways to a sequence that does not capture their full extent, biological relevance, complexity, and temporal dimension.
Moreover, our concept is purely abstract and not capable of quantification or forecasting expected changes. It is a skeleton that needs to be filled with life. Further, it has proven difficult to find an unambiguous language and visualisation for the concept. For example, we depict organisms that are a pool and organisms that are agents with the same symbol. Adjustments of terms and symbols appear reasonable as soon as users identify problems. We hope that the concept will grow, improve, and become more complete with use.
We propose a general (visual) concept for the marine part of the organic carbon cycle. It complements and integrates existing concepts and defines overarching OC structures such as remineralisation and rDOC loops and the processes, pools, and agents involved. Building on concepts that focus on individual or a subset of marine OC pathways, our concept identifies general structures of all pathways. Details and complexity are disregarded in favour of systematic structures that can facilitate the identification and comparison of concepts, pathways, and pools. The concept can be adapted to a wide range of questions, pathway choices, and resolutions, and thus serve as a basis for discussion and reference to understand current and future marine OC dynamics arising from the multiplicity of OC pathways and the human influence on them.
The literature-based pathway concept is attached in the Supplement S1.
The supplement related to this article is available online at: https://doi.org/10.5194/os-18-437-2022-supplement.
MIES designed the study, conducted the research, and prepared the manuscript. IH designed the study and contributed to the manuscript.
The contact author has declared that neither they nor their co-author has any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Our special thanks go to Laurin Steidle, Alex Kitts, Felix Pellerin, Rémy Asselot, Isabell Hochfeld, Josefine Herrford, and Jana Hinners for their valuable feedback and Scott Dorssers for his support in finalising the literature-based pathway concept. We thank one anonymous referee and Gwenaelle Gremion for their helpful criticism and comments.
This study has been supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy - EXC 2037 “Climate, Climatic Change, and Society” (project no. 390683824), a contribution to the Center for Earth System Research and Sustainability (CEN) of Universität Hamburg.
This paper was edited by Anne Marie Tréguier and reviewed by Gwenaelle Gremion and one anonymous referee.
Anderson, T. R. and Ducklow, H. W.: Microbial loop carbon cycling in ocean environments studied using a simple steady-state model, Aquat. Microb. Ecol., 26, 37–49, https://doi.org/10.3354/ame026037, 2001. a
Arrieta, J. M., Mayol, E., Hansman, R. L., Herndl, G. J., Dittmar, T., and Duarte, C. M.: Ocean chemistry. Dilution limits dissolved organic carbon utilization in the deep ocean, Science, 348, 331–333, https://doi.org/10.1126/science.1258955, 2015. a
Azam, F. and Malfatti, F.: Microbial structuring of marine ecosystems, Nature reviews, Microbiology, 5, 782–791, https://doi.org/10.1038/nrmicro1747, 2007. a
Azam, F., Smith, D. C., Steward, G. F., and Hagström, A.: Bacteria-organic matter coupling and its significance for oceanic carbon cycling, Microb. Ecol., 28, 167–179, https://doi.org/10.1007/BF00166806, 1994. a
Baker, C. A., Henson, S. A., Cavan, E. L., Giering, S. L. C., Yool, A., Gehlen, M., Belcher, A., Riley, J. S., Smith, H. E. K., and Sanders, R.: Slow-sinking particulate organic carbon in the Atlantic Ocean: Magnitude, flux, and potential controls, Global Biogeochem. Cy., 31, 1051–1065, https://doi.org/10.1002/2017GB005638, 2017. a
Barange, M., Butenschön, M., Yool, A., Beaumont, N., Fernandes, J. A., Martin, A. P., and Allen, J. I.: The Cost of Reducing the North Atlantic Ocean Biological Carbon Pump, Front. Mar. Sci., 3, 1–10, https://doi.org/10.3389/fmars.2016.00290, 2017. a
Benner, R. and Biddanda, B.: Photochemical transformations of surface and deep marine dissolved organic matter: Effects on bacterial growth, Limnol. Oceanogr., 43, 1373–1378, 1998. a
Berke, S. K.: Functional groups of ecosystem engineers: a proposed classification with comments on current issues, Integr. Comp. Biol., 50, 147–157, https://doi.org/10.1093/icb/icq077, 2010. a
Bianchi, D., Carozza, D. A., Galbraith, E. D., Guiet, J., and DeVries, T.: Estimating global biomass and biogeochemical cycling of marine fish with and without fishing, Sci. Adv., 7, eabd7554, https://doi.org/10.1126/sciadv.abd7554, 2021. a
Boscolo-Galazzo, F., Crichton, K. A., Barker, S., and Pearson, P. N.: Temperature dependency of metabolic rates in the upper ocean: A positive feedback to global climate change?, Glob. Planet. Change, 170, 201–212, https://doi.org/10.1016/j.gloplacha.2018.08.017, 2018. a, b
Boudreau, B. P.: Mean mixed depth of sediments: The wherefore and the why, Limnol. Oceanogr., 43, 524–526, https://doi.org/10.4319/lo.1998.43.3.0524, 1998. a
Boyd, P. W., Claustre, H., Levy, M., Siegel, D. A., and Weber, T.: Multi-faceted particle pumps drive carbon sequestration in the ocean, Nature, 568, 327–335, https://doi.org/10.1038/s41586-019-1098-2, 2019. a, b
Briggs, N., Dall'Olmo, G., and Claustre, H.: Major role of particle fragmentation in regulating biological sequestration of CO2 by the oceans, Science, 367, 791–793, https://doi.org/10.1126/science.aay1790, 2020. a
Buchan, A., LeCleir, G. R., Gulvik, C. A., and González, J. M.: Master recyclers: features and functions of bacteria associated with phytoplankton blooms, Nature reviews, Microbiology, 12, 686–698, https://doi.org/10.1038/nrmicro3326, 2014. a
Bunke, D., Leipe, T., Moros, M., Morys, C., Tauber, F., Virtasalo, J. J., Forster, S., and Arz, H. W.: Natural and Anthropogenic Sediment Mixing Processes in the South-Western Baltic Sea, Front. Mar. Sci., 6, 1–20, https://doi.org/10.3389/fmars.2019.00677, 2019. a
Burdige, D. J., Berelson, W. M., Coale, K. H., McManus, J., and Johnson, K. S.: Fluxes of dissolved organic carbon from California continental margin sediments, Geochim. Cosmochim. Ac., 63, 1507–1515, https://doi.org/10.1016/S0016-7037(99)00066-6, 1999. a
Carlson, C. A. and Hansell, D. A.: Chap. 3 – DOM Sources, Sinks, Reactivity, and Budgets, in: Biogeochemistry of marine dissolved organic matter, edited by: Carlson, C. A., Hansell, D. A., and Amon, R. M. W., Academic Press, London, 65–126, https://doi.org/10.1016/B978-0-12-405940-5.00003-0, 2015. a
Cavan, E. L., Belcher, A., Atkinson, A., Hill, S. L., Kawaguchi, S., McCormack, S., Meyer, B., Nicol, S., Ratnarajah, L., Schmidt, K., Steinberg, D. K., Tarling, G. A., and Boyd, P. W.: The importance of Antarctic krill in biogeochemical cycles, Nat. Commun., 10, 4742, https://doi.org/10.1038/s41467-019-12668-7, 2019. a
Dai, M., Yin, Z., Meng, F., Liu, Q., and Cai, W.-J.: Spatial distribution of riverine DOC inputs to the ocean: an updated global synthesis, Curr. Opin. Environ. Sustain., 4, 170–178, https://doi.org/10.1016/j.cosust.2012.03.003, 2012. a
De La Rocha, C. L.: The Biological Pump, in: The oceans and marine geochemistry, edited by: Elderfield, H. and Holland, H. D., Treatise on geochemistry, Elsevier, Amsterdam, ISBN 9780080451015, 2006. a, b, c
DeVries, T., Primeau, F., and Deutsch, C.: The sequestration efficiency of the biological pump, Geophys. Res. Lett., 39, 1–5, https://doi.org/10.1029/2012GL051963, 2012. a
Dickey, T. D.: Physical-optical-biological scales relevant to recruitment in large marine ecosystems, Large marine ecosystems: Patterns, processes, and yields, edited by: Sherman, K., Alexander, LM, and Gold, BD, Am. Assoc. Adv. Sci. Publ, 90, 82–98, 1990. a
Dilling, L. and Alldredge, A. L.: Fragmentation of marine snow by swimming macrozooplankton: A new process impacting carbon cycling in the sea, Deep-Sea Res. Pt. I, 47, 1227–1245, https://doi.org/10.1016/S0967-0637(99)00105-3, 2000. a
Doney, S. C. and Ducklow, H. W.: A decade of synthesis and modeling in the US Joint Global Ocean Flux Study, Deep-Sea Res. Pt. II, 53, 451–458, https://doi.org/10.1016/j.dsr2.2006.01.019, 2006. a
Duarte, C. M. and Cebrián, J.: The fate of marine autotrophic production, Limnol. Oceanogr., 41, 1758–1766, https://doi.org/10.4319/lo.1996.41.8.1758, 1996. a
Fortuin, K. P. J., van Koppen, C. S. A., and Leemans, R.: The Value of Conceptual Models in Coping with Complexity and Interdisciplinarity in Environmental Sciences Education, BioScience, 61, 802–814, https://doi.org/10.1525/bio.2011.61.10.10, 2011. a
Gardner, W. D., Chung, S. P., Richardson, M. J., and Walsh, I. D.: The oceanic mixed-layer pump, Deep-Sea Res. Pt. II, 42, 757–775, https://doi.org/10.1016/0967-0645(95)00037-Q, 1995. a
Giering, S. L. C. and Humphreys, M. P.: Biological Pump, in: Encyclopedia of Engineering Geology, edited by Bobrowsky, P. T. and Marker, B., Encyclopedia of Earth Sciences Series, Springer International Publishing, Cham, 1–6, https://doi.org/10.1007/978-3-319-39193-9_154-1, 2020. a, b, c, d, e, f
Giering, S. L. C., Sanders, R., Lampitt, R. S., Anderson, T. R., Tamburini, C., Boutrif, M., Zubkov, M. V., Marsay, C. M., Henson, S. A., Saw, K., Cook, K., and Mayor, D. J.: Reconciliation of the carbon budget in the ocean's twilight zone, Nature, 507, 480–483, https://doi.org/10.1038/nature13123, 2014. a
Gnanadesikan, A. and Marinov, I.: Export is not enough: nutrient cycling and carbon sequestration, Mar. Ecol. Prog. Ser., 364, 289–294, https://doi.org/10.3354/meps07550, 2008. a
Goldthwait, S., Yen, J., Brown, J., and Alldredge, A.: Quantification of marine snow fragmentation by swimming euphausiids, Limnol. Oceanogr., 49, 940–952, https://doi.org/10.4319/lo.2004.49.4.0940, 2004. a
Griffiths, J. R., Kadin, M., Nascimento, F. J. A., Tamelander, T., Törnroos, A., Bonaglia, S., Bonsdorff, E., Brüchert, V., Gårdmark, A., Järnström, M., Kotta, J., Lindegren, M., Nordström, M. C., Norkko, A., Olsson, J., Weigel, B., Žydelis, R., Blenckner, T., Niiranen, S., and Winder, M.: The importance of benthic-pelagic coupling for marine ecosystem functioning in a changing world, Glob. Change Biol., 23, 2179–2196, https://doi.org/10.1111/gcb.13642, 2017. a
Halsey, K. H., Giovannoni, S. J., Graus, M., Zhao, Y., Landry, Z., Thrash, J. C., Vergin, K. L., and de Gouw, J.: Biological cycling of volatile organic carbon by phytoplankton and bacterioplankton, Limnol. Oceanogr., 62, 2650–2661, https://doi.org/10.1002/lno.10596, 2017. a
Hansell, D., Carlson, C., Repeta, D., and Schlitzer, R.: Dissolved Organic Matter in the Ocean: A Controversy Stimulates New Insights, Oceanography, 22, 202–211, https://doi.org/10.5670/oceanog.2009.109, 2009. a
Hansell, D. A.: Recalcitrant dissolved organic carbon fractions, Ann. Rev. Mar. Sci., 5, 421–445, https://doi.org/10.1146/annurev-marine-120710-100757, 2013. a, b, c, d
Hansell, D. A. and Carlson, C. A.: Marine dissolved organic matter and the carbon cycle, Oceanography, 14, 41–49, 2001. a, b
Heemskerk, M., Wilson, K., and Pavao-Zuckerman, M.: Conceptual models as tools for communication across disciplines, Conserv. Ecol., 7, 8, https://doi.org/10.5751/ES-00554-070308, 2003. a
Honjo, S., Eglinton, T., Taylor, C., Ulmer, K., Sievert, S., Bracher, A., German, C., Edgcomb, V., Francois, R., Iglesias-Rodriguez, M. D., van Mooy, B., and Rapeta, D.: Understanding the Role of the Biological Pump in the Global Carbon Cycle: An Imperative for Ocean Science, Oceanography, 27, 10–16, https://doi.org/10.5670/oceanog.2014.78, 2014. a
Huntley, M. E. and Zhou, M.: Influence of animals on turbulence in the sea, Mar. Ecol. Prog. Ser., 273, 65–79, https://doi.org/10.3354/meps273065, 2004. a
Jiao, N. and Zheng, Q.: The Microbial Carbon Pump: from Genes to Ecosystems, Appl. Environ. Microbiol., 77, 7439–7444, https://doi.org/10.1128/AEM.05640-11, 2011. a, b, c, d, e, f, g
Jiao, N., Herndl, G. J., Hansell, D. A., Benner, R., Kattner, G., Wilhelm, S. W., Kirchman, D. L., Weinbauer, M. G., Luo, T., Chen, F., and Azam, F.: Microbial production of recalcitrant dissolved organic matter: long-term carbon storage in the global ocean, Nature reviews, Microbiology, 8, 593–599, https://doi.org/10.1038/nrmicro2386, 2010. a, b, c
Jiao, N., Guo, Z., Legendre, L., Suttle, C., Rivkin, R., and Azam, F.: Editorial for the special issue on marine carbon sequestration and climate change, Nat. Sci. Rev., 5, 456–457, https://doi.org/10.1093/nsr/nwy068, 2018. a
Jónasdóttir, S. H., Visser, A. W., Richardson, K., and Heath, M. R.: Seasonal copepod lipid pump promotes carbon sequestration in the deep North Atlantic, P. Natl. Acad. Sci. USA, 112, 12122–12126, https://doi.org/10.1073/pnas.1512110112, 2015. a
Katija, K. and Dabiri, J. O.: A viscosity-enhanced mechanism for biogenic ocean mixing, Nature, 460, 624–626, https://doi.org/10.1038/nature08207, 2009. a
Kharbush, J. J., Close, H. G., van Mooy, B. A. S., Arnosti, C., Smittenberg, R. H., Le Moigne, F. A. C., Mollenhauer, G., Scholz-Böttcher, B., Obreht, I., Koch, B. P., Becker, K. W., Iversen, M. H., and Mohr, W.: Particulate Organic Carbon Deconstructed: Molecular and Chemical Composition of Particulate Organic Carbon in the Ocean, Front. Mar. Sci., 7, 1–10, https://doi.org/10.3389/fmars.2020.00518, 2020. a, b
Kieber, D. J., McDaniel, J., and Mopper, K.: Photochemical source of biological substrates in sea water: implications for carbon cycling, Nature, 341, 637–639, https://doi.org/10.1038/341637a0, 1989. a, b
Kristensen, E., Penha-Lopes, G., Delefosse, M., Valdemarsen, T., Quintana, C. O., and Banta, G. T.: What is bioturbation? The need for a precise definition for fauna in aquatic sciences, Mar. Ecol. Prog. Ser., 446, 285–302, https://doi.org/10.3354/meps09506, 2012. a
Kunze, E., Dower, J. F., Beveridge, I., Dewey, R., and Bartlett, K. P.: Observations of biologically generated turbulence in a coastal inlet, Science, 313, 1768–1770, https://doi.org/10.1126/science.1129378, 2006. a
Lampert, W.: Release of dissolved organic carbon by grazing zooplankton, Limnol. Oceanogr., 23, 831–834, https://doi.org/10.4319/lo.1978.23.4.0831, 1978. a
Lampitt, R. S., Noji, T., and von Bodungen, B.: What happens to zooplankton faecal pellets? Implications for material flux, Mar. Biol., 104, 15–23, https://doi.org/10.1007/BF01313152, 1990. a
Lang, S. Q., Butterfield, D. A., Lilley, M. D., Paul Johnson, H., and Hedges, J. I.: Dissolved organic carbon in ridge-axis and ridge-flank hydrothermal systems, Geochim. Cosmochim. Ac., 70, 3830–3842, https://doi.org/10.1016/j.gca.2006.04.031, 2006. a
Legendre, L., Rivkin, R. B., Weinbauer, M. G., Guidi, L., and Uitz, J.: The microbial carbon pump concept: Potential biogeochemical significance in the globally changing ocean, Prog. Oceanogr., 134, 432–450, https://doi.org/10.1016/j.pocean.2015.01.008, 2015. a
Lenhart, K., Klintzsch, T., Langer, G., Nehrke, G., Bunge, M., Schnell, S., and Keppler, F.: Evidence for methane production by the marine algae Emiliania huxleyi, Biogeosciences, 13, 3163–3174, https://doi.org/10.5194/bg-13-3163-2016, 2016. a
Levy, M., Bopp, L., Karleskind, P., Resplandy, L., Ethe, C., and Pinsard, F.: Physical pathways for carbon transfers between the surface mixed layer and the ocean interior, Global Biogeochem. Cy., 27, 1001–1012, https://doi.org/10.1002/gbc.20092, 2013. a
Margoluis, R., Stem, C., Salafsky, N., and Brown, M.: Using conceptual models as a planning and evaluation tool in conservation, Eval. Program Plann., 32, 138–147, https://doi.org/10.1016/j.evalprogplan.2008.09.007, 2009. a
Martin, A. H., Pearson, H. C., Saba, G. K., and Olsen, E. M.: Integral functions of marine vertebrates in the ocean carbon cycle and climate change mitigation, One Earth, 4, 680–693, https://doi.org/10.1016/j.oneear.2021.04.019, 2021. a
Mayer, L. M., Schick, L. L., Skorko, K., and Boss, E.: Photodissolution of particulate organic matter from sediments, Limnol. Oceanogr., 51, 1064–1071, https://doi.org/10.4319/lo.2006.51.2.1064, 2006. a
Mayer, L. M., Schick, L. L., Hardy, K. R., and Estapa, M. L.: Photodissolution and other photochemical changes upon irradiation of algal detritus, Limnol. Oceanogr., 54, 1688–1698, https://doi.org/10.4319/lo.2009.54.5.1688, 2009. a
McClain, C. R., Nunnally, C., Dixon, R., Rouse, G. W., and Benfield, M.: Alligators in the abyss: The first experimental reptilian food fall in the deep ocean, PloS one, 14, e0225 345, https://doi.org/10.1371/journal.pone.0225345, 2019. a
Middelboe, M., Jorgensen, N., and Kroer, N.: Effects of viruses on nutrient turnover and growth efficiency of noninfected marine bacterioplankton, Appl. Environ. Microbiol., 62, 1991–1997, https://doi.org/10.1128/aem.62.6.1991-1997.1996, 1996. a
Middelburg, J. J.: Carbon Processing at the Seafloor, in: Marine Carbon Biogeochemistry, edited by: Middelburg, J. J., SpringerBriefs in Earth System Sciences, Springer International Publishing, Cham, 57–75, https://doi.org/10.1007/978-3-030-10822-9_4, 2019. a, b, c
Mopper, K. and Kieber, D. J.: Photochemistry and the cycling of carbon, sulfer, nitrogen and phosphorus, in: Biogeochemistry of marine dissolved organic matter, edited by: Hansell, D. A. and Carlson, C. A., Academic Press, Amsterdam and Boston, 455–508, ISBN 9780123238412, 2002. a
Oka, A.: Ocean carbon pump decomposition and its application to CMIP5 earth system model simulations, Prog. Earth Planet. Sci., 7, 1–17, https://doi.org/10.1186/s40645-020-00338-y, 2020. a
Omand, M. M., D'Asaro, E. A., Lee, C. M., Perry, M. J., Briggs, N., Cetinić, I., and Mahadevan, A.: Eddy-driven subduction exports particulate organic carbon from the spring bloom, Science, 348, 222–225, https://doi.org/10.1126/science.1260062, 2015. a
Passow, U. and Carlson, C. A.: The biological pump in a high CO2 world, Mar. Ecol. Prog. Ser., 470, 249–271, https://doi.org/10.3354/meps09985, 2012. a
Pedersen, O., Sand-Jensen, K., and Revsbech, N. P.: Diel Pulses of O2 and CO2 in Sandy Lake Sediments Inhabited by Lobelia Dortmanna, Ecology, 76, 1536–1545, https://doi.org/10.2307/1938155, 1995. a
Roman, J. and McCarthy, J. J.: The whale pump: marine mammals enhance primary productivity in a coastal basin, PloS One, 5, e13255, https://doi.org/10.1371/journal.pone.0013255, 2010. a
Roshan, S. and DeVries, T.: Efficient dissolved organic carbon production and export in the oligotrophic ocean, Nat. Commun., 8, 2036, https://doi.org/10.1038/s41467-017-02227-3, 2017. a
Rowe, G. T. and Deming, J. W.: An alternative view of the role of heterotrophic microbes in the cycling of organic matter in deep-sea sediments, Mar. Biol. Res., 7, 629–636, https://doi.org/10.1080/17451000.2011.560269, 2011. a
Ruiz, J.: What generates daily cycles of marine snow?, Deep-Sea Res. Pt. I, 44, 1105–1126, https://doi.org/10.1016/S0967-0637(97)00012-5, 1997. a
Scheiner, S. M. and Willig, M. R.: The theory of ecology, University of Chicago Press, ISBN 0226736865, 2011. a, b, c
Schmale, O., Wäge, J., Mohrholz, V., Wasmund, N., Gräwe, U., Rehder, G., Labrenz, M., and Loick-Wilde, N.: The contribution of zooplankton to methane supersaturation in the oxygenated upper waters of the central Baltic Sea, Limnol. Oceanogr., 63, 412–430, 2018. a
Shen, Y. and Benner, R.: Mixing it up in the ocean carbon cycle and the removal of refractory dissolved organic carbon, Sci. Rep., 8, 2542, https://doi.org/10.1038/s41598-018-20857-5, 2018. a
Sigman, D. M. and Haug, G. H.: 6.18 – The Biological Pump in the Past, in: 6: The oceans and marine geochemistry, edited by: Elderfield, H., Elsevier, Amsterdam, 491–528, https://doi.org/10.1016/B0-08-043751-6/06118-1, 2004. a
Smith, D. C., Simon, M., Alldredge, A. L., and Azam, F.: Intense hydrolytic enzyme activity on marine aggregates and implications for rapid particle dissolution, Nature, 359, 139–142, https://doi.org/10.1038/359139a0, 1992. a
Steinberg, D. K. and Landry, M. R.: Zooplankton and the Ocean Carbon Cycle, Ann. Rev. Mar. Sci., 9, 413–444, https://doi.org/10.1146/annurev-marine-010814-015924, 2017. a, b
Steinberg, D. K., Goldthwait, S. A., and Hansell, D. A.: Zooplankton vertical migration and the active transport of dissolved organic and inorganic nitrogen in the Sargasso Sea, Deep-Sea Res. Pt. I, 49, 1445–1461, https://doi.org/10.1016/S0967-0637(02)00037-7, 2002. a
Stoderegger, K. and Herndl, G. J.: Production and release of bacterial capsular material and its subsequent utilization by marine bacterioplankton, Limnol. Oceanogr., 43, 877–884, https://doi.org/10.4319/lo.1998.43.5.0877, 1998. a, b
Ullah, H., Nagelkerken, I., Goldenberg, S. U., and Fordham, D. A.: Climate change could drive marine food web collapse through altered trophic flows and cyanobacterial proliferation, PLoS Biol., 16, e2003446, https://doi.org/10.1371/journal.pbio.2003446, 2018. a
Weber, T., Wiseman, N. A., and Kock, A.: Global ocean methane emissions dominated by shallow coastal waters, Nat. Commun., 10, 4584, https://doi.org/10.1038/s41467-019-12541-7, 2019. a
Wooster, M. K., McMurray, S. E., Pawlik, J. R., Morán, X. A. G., and Berumen, M. L.: Feeding and respiration by giant barrel sponges across a gradient of food abundance in the Red Sea, Limnol. Oceanogr., 64, 1790–1801, https://doi.org/10.1002/lno.11151, 2019. a
If not mentioned differently, we always refer to the BCP definition by Giering and Humphreys (2020) in the following discussion.