the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
The diurnal cycle of pCO2 in the coastal region of the Baltic Sea
Jens Daniel Müller
Jukka Seppälä
Gregor Rehder
Sami Kielosto
Pasi Ylöstalo
Timo Mäkelä
Juha Hatakka
Lauri Laakso
The direction and magnitude of carbon dioxide fluxes between the atmosphere and the sea are regulated by the gradient in the partial pressure of carbon dioxide (pCO2) across the air–sea interface. Typically, observations of pCO2 at the sea surface are carried out by using research vessels and ships of opportunity, which usually do not resolve the diurnal cycle of pCO2 at a given location. This study evaluates the magnitude and driving processes of the diurnal cycle of pCO2 in a coastal region of the Baltic Sea. We present pCO2 data from July 2018 to June 2019 measured in the vicinity of the island of Utö at the outer edge of the Archipelago Sea, and quantify the relevant physical, biological, and chemical processes controlling pCO2. The highest monthly median of diurnal pCO2 variability (31 µatm) was observed in August and predominantly driven by biological processes. Biological fixation and mineralization of carbon led to sinusoidal diurnal pCO2 variations, with a maximum in the morning and a minimum in the afternoon. Compared with the biological carbon transformations, the impacts of air–sea fluxes and temperature changes on pCO2 were small, with their contributions to the monthly medians of diurnal pCO2 variability being up to 12 and 5 µatm, respectively. During upwelling events, short-term pCO2 variability (up to 500 µatm within a day) largely exceeded the usual diurnal cycle. If the net annual air–sea flux of carbon dioxide at our study site and for the sampled period is calculated based on a data subset that consists of only one regular measurement per day, the bias in the net exchange depends on the sampling time and can amount up to ±12 %. This finding highlights the importance of continuous surface pCO2 measurements at fixed locations for the assessment of the short-term variability of the carbonate system and the correct determination of air–sea CO2 fluxes.
- Article
(2088 KB) - Full-text XML
- BibTeX
- EndNote
Over the last decade (2009–2018), anthropogenic carbon dioxide (CO2) emissions to the atmosphere amounted to 11 gigatons of carbon per year, mainly driven by fossil fuel combustion, land use change, and cement production. Approximately half of these emissions were bound by the terrestrial biosphere (3.2 GtC yr−1) and the oceans (2.5 GtC yr−1) together (Friedlingstein et al., 2019). The increased CO2 concentration in the atmosphere causes global warming, while the increase of CO2 dissolved in the oceans drives ocean acidification (Feely et al., 2009). The correct quantification of air–sea fluxes of CO2 is thus an essential component to keep track of the redistribution of anthropogenic carbon within the Earth system and assess its potentially harmful impact. The air–sea CO2 fluxes can undergo large daily variations, and thus it is vital to understand the daily dynamics of the processes driving the flux in order to provide an accurate estimate of the net annual air–sea CO2 fluxes.
The partial pressure of CO2 (pCO2) in surface seawater and thus the direction of the air–sea CO2 flux (Fas) are mainly regulated by the interplay of biological productivity and respiration, temperature-induced changes in seawater carbonate chemistry, and mixing processes. As the sea surface receives more solar radiation during the day than at night, a diurnal cycle in the biology, physics, and chemistry of the surface seawater is established. Since sea surface pCO2 observations are widely used for calculating the CO2 exchange between the sea and the atmosphere, there can be large discrepancies between the flux estimates when using pCO2 values measured at different times of the day.
The diurnal variation of the pCO2 is typically larger in coastal seas than in the open oceans due to the larger biological activity. Goyet and Peltzer (1997) found that the diurnal cycle of pCO2 in open ocean conditions was 8 µatm. The diurnal pCO2 cycle has been studied, e.g., in an oligotrophic ocean (Olsen et al., 2004), at coral reefs (Yan et al., 2016), and in tidal regions (Andersson and Mackenzie, 2012). A limited number of studies have addressed the diurnal cycle of pCO2 in the Baltic Sea. Lansø et al. (2017) found that there was no evident diurnal pCO2 signal in the Baltic Proper and Arkona Basin in wintertime, but during April–October, the monthly average pCO2 amplitudes were up to 27 µatm. Wesslander et al. (2011) determined that the diurnal pCO2 variability in the Baltic Proper was mainly controlled by biological processes, mixing, or the air–sea exchange of CO2. Huge (up to 1604 µatm) diurnal variability of pCO2 in a highly productive macrophyte meadow in the western Baltic Sea was reported by Saderne et al. (2013).
The carbon system of the Baltic Sea shows large spatial variability. On the one hand, the northern part of the Baltic Sea, i.e., the Gulf of Bothnia, is characterized by large fluvial fluxes of organic matter into its basins, which turns the area into a source of CO2 for the atmosphere through effective bacterial remineralization (Algesten et al., 2006). On the other hand, the southern parts of the Baltic Sea exhibit larger primary production compared with the Gulf of Bothnia (Wasmund et al., 2001), a larger input of alkalinity from land, and lower input of organic matter, which makes the basin act as a carbon sink (Kuliński and Pempkowiak, 2011). Based on the mass balance approach of Kuliński and Pempkowiak (2011), revisited by Ylöstalo et al. (2016), the Baltic Sea as a whole is considered to be a weak source of carbon dioxide for the atmosphere.
Measurements of pCO2 taken by ships of opportunity (SOOPs) have proved to be a cost-effective method to reveal new insights into the spatiotemporal variability of the Baltic Sea's carbon cycle (Schneider et al., 2014; Schneider and Müller, 2018). These surface pCO2 measurements carried out on SOOP routes are currently our best presentation of the spatial variability of CO2 partial pressure in the Baltic Sea. However, the measurements carried out on these fixed routes and time schedules do not resolve the diurnal cycle, and when interpreting these data, one should consider the potential bias caused by the time of the sampling. Fixed stationary platforms, though limited in their spatial coverage, are capable of measuring in high temporal resolution and resolving the diurnal cycle of pCO2 and thus provide data that are highly complementary to data retrieved on SOOPs or research vessels (RVs).
In this contribution, we investigate the diurnal cycle of the carbon dioxide system at a fixed station near the island of Utö, located in the transition zone between the northern Baltic Proper and the Archipelago Sea, representing a highly productive (euthrophied) coastal ecosystem. The aims of this study are (a) to investigate the diurnal cycle of pCO2 during different seasons based on observations carried out at Utö and (b) to quantify the contributions of the main drivers and processes affecting the pCO2 diurnal variations: air–sea flux, biological carbon uptake and release, and diurnal changes in temperature.
2.1 Study site
The Utö Atmospheric and Marine Research Station is located on the island of Utö (Fig. 1) on the southern edge of the Archipelago Sea (59∘46′55′′ N, 21∘21′27′′ E). Utö is a small (0.81 km2) rocky island with low vegetation.
As characteristic for the central Baltic Sea, our study site is affected by a climate-change-induced increase of seawater temperature (Laakso et al., 2018). Besides the warming trend, also stratification has strengthened, affecting the connectivity between water layers separated by a seasonal thermocline and a permanent halocline (Liblik and Lips, 2019). Long-term trends of increasing alkalinity throughout the Baltic Sea have been shown to partly compensate acidification induced by rising atmospheric CO2 (Müller et al., 2016). Within our study region, phytoplankton blooms are a recurrent phenomenon due to eutrophication (Kraft et al., 2021).
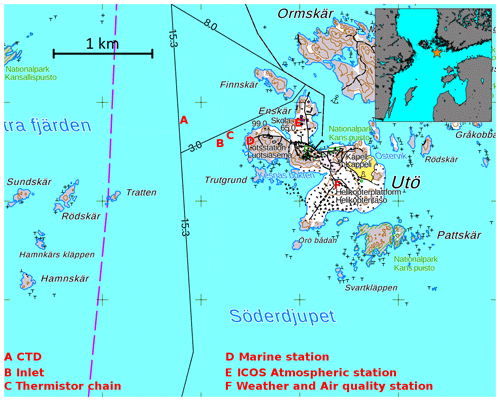
Figure 1Sampling locations at Utö Atmospheric and Marine Research Station. The grid size (distance between plus signs) is 1 km. The smaller figure in the upper right corner shows the location of Utö (orange star). The National Land Survey of Finland is acknowledged for providing the map.
The marine observations at the station focus on regional marine ecosystem functioning with a large number of biochemical and physical observations. The marine observations include, but are not limited to, conductivity–temperature–depth (CTD) casts carried out northwest from the island, flow-through analyses at the marine station and thermistor measurements in the vicinity of the seawater inlet (Fig. 1). The measurements of the Utö Atmospheric and Marine Research Station belong to the Joint European Research Infrastructure for Coastal Observatories (JERICO-RI, http://www.jerico-ri.eu, last access: 5 November 2021). Carbonate system dynamics is noted as one of the key scientific topics in coastal ocean studies (Farcy et al., 2019), and the study presented here, executed under the framework of the JERICO-RI, highlights the need for integrated and multidisciplinary observations. The atmospheric part of the station includes a wide range of meteorological, greenhouse gas and aerosol measurements. The micrometeorological flux tower at the western shore, next to the marine station, measures the CO2, sensible heat, and latent heat fluxes between the sea and the atmosphere. Greenhouse gas monitoring and some meteorological measurements are part of the Integrated Carbon Observation System Research Infrastructure (ICOS RI). For the complete list of observations, visit the Finnish Meteorological Institute's website (https://en.ilmatieteenlaitos.fi/uto-observations, last access: 5 November 2021). Site bathymetry and other information about the study site are given in Laakso et al. (2018) and Kraft et al. (2021). Our study is based on 1 year of data gathered between July 2018 and June 2019. The timing of all data presented in this paper is given in UTC. Finland belongs to the UTC+02:00 time zone.
2.2 Flow-through sampling
The marine station, located on the western shore of the island (Fig. 1), is equipped with a flow-through system. A submersible pump located 250 m from the shore transports seawater from the inlet to the marine station, where seawater is analyzed automatically or manually on demand. The bottom-moored floating seawater inlet is at the approximate depth of 4.5 m ± 0.5 m, directly above the submersible pump. The mean depth at this location is 23 m and the sea level at Utö varies ±0.5 m relative to theoretical mean sea level. At the location, there are no notable tides or tidal currents.
At the station, the transported water first enters a manifold. Any flow-through instrument can be attached to the manifold separately, enabling individual adjustment of the flow rate for each instrument. The time stamp of the flow-through data is shifted (5.6 min on average) according to the concurrent flow rate (54–68 L min−1) to match the time of sampling at the intake, based on the known volume of the pipe system.
All of the instruments attached to the flow-through system are automatically washed with cleaning fluid (hydrogen peroxide or Triton X-100) daily. The data gathered during and immediately after the cleaning have been discarded.
2.2.1 Measurement of pCO2
A SuperCO2 instrument (Sunburst Sensors), which was connected to the flow-through system, was used to measure pCO2. In its two shower-head equilibrator chambers, the seawater CO2 is equilibrated with the gas above according to Henry's law (Eq. B2). The equilibrated gas is analyzed for its CO2 molar fraction (xCO2) by an infrared gas analyzer (LI-840A, LI-COR). The logging interval was 10–15 s.
The sensor drift of the gas analyzer is taken into account by measuring four standard gases with differing CO2 molar fractions (0.00, 234.38, 396.69, and 993.45 ppm, ±2 %) every fourth hour in order to form a correction equation for dry pCO2. The Finnish Meteorological Institute (FMI) buys the reference gases from the Finnish branch of Linde-Gas (previously AGA). The gas concentrations are checked with instruments using cavity ring-down spectroscopy in the FMI laboratory prior to measurements. These instruments are calibrated using gases that are verified by the National Oceanic and Atmospheric Administration (USA). Aluminum gas containers have been used in order to minimize the concentration drift.
Drift-corrected dry xCO2 is transformed into pCO2 as described in Dickson et al. (2007), with a slight modification. Since the water trap attached to the sample gas line may slightly affect the water vapor content, the following calculation was used. The dry CO2 molar fraction was calculated using the H2O measured using the analyzer. The real water vapor content in the equilibrium chambers was calculated using the temperature and salinity data assuming full saturation. This real water vapor content, together with the dry xCO2, was used when calculating the partial pressure of CO2.
During May–June 2019, the sampling and inlet tube system was tested by measuring pCO2 with two SAMI2 sensors (Sunburst Sensors) that were parallel to the SuperCO2 system inside the measurement station on land (20–23 May 2019), followed by deployment of the SAMI2 sensors next to the sampling inlet at sea (from 24 May to 7 June 2019). The parallel measurement inside the station was used to correct the potential initial offset of the SAMI2 sensors against the SuperCO2 system. While the SAMI2 sensors were positioned close to the inlet at sea, the in situ concentrations for all three instruments closely followed each other: the root mean square difference between measurements at the sea inlet and the station was 4.1 µatm. We conclude that the pCO2 analysis carried out in the station, despite the unusually long path of water from the inlet location to the lab, fully represent the conditions at the inlet.
2.2.2 Other flow-through measurements
The equilibrator temperature (together with salinity) was measured using a thermosalinograph (SBE45 MicroTSG, Sea-Bird Scientific) next to the SuperCO2 instrument. The thermosalinograph is cleaned one to two times a year. The accuracies for temperature and salinity given by the manufacturer are, respectively, 0.002 ∘C and 0.005. The temperature drift is less than a few thousandths of a degree per year, whereas the stability of conductivity measurement depends mostly on the cleanliness of the measurement cell. The thermosalinograph logged data every 15 s.
Oxygen was measured with an oxygen optode (Aanderaa 4330) with multipoint calibration. The optode has a preburned foil providing long-term stability. The accuracy of the optode is 2 µM according to the manufacturer. For the work presented here, we are mostly interested in hourly changes of oxygen, and thus the drift of the absolute value is not of concern. Chlorophyll a was measured with a Wetlabs fluorometer and turbidity meter (FLNTU), as a proxy of chlorophyll concentration, using factory calibration. Both were connected to the flow-through system. Chlorophyll a measurement was offline in winter (January–March). Both instruments logged data every 15 s.
2.3 Measurements from other sampling locations
2.3.1 Hydrographic measurements
The vertical temperature profiles were measured with temperature chains, supported with regular interval profiles of the CTD instrument, RBR XR-620. The CTD profiles were taken fortnightly by using a small boat during the productive period and with lower temporal resolution in winter (see Fig. 2a). The CTD location is approximately 400 m west of the sampling inlet.
The thermistor chain was deployed 150 m northeast from the seawater inlet in July 2018; this chain was moored at the depth of 21.3 m ± 0.5 m, and its Pt-100 thermistors were placed at the heights of approximately 18, 13, 8, 1, and 0 m from the bottom (depths 3.3 m ± 0.5 m, 8.3 m ± 0.5 m, 13.3 m ± 0.5 m, 20.3 m ± 0.5 m, 21.3 m ± 0.5 m). In order to avoid instrument damages during rough weather conditions, there was no thermistors closer than 3 m to the surface. Pt-100 thermistors were calibrated prior to the deployment in FMI's laboratory, and the maximum error in temperature was found to be less than 0.015 ∘C. Thermistors logged data every 30 s.
The thermistor profiles were used to verify that the CTD casts, carried out at a slightly different location, were representative of the hydrographic conditions at the seawater inlet. More importantly, the 3 m thermistor measurement was used as in situ temperature at the inlet, and hence for correcting the pCO2 for the temperature difference between in situ conditions and in the equilibration chamber.
2.3.2 Atmospheric CO2 measurement
The atmospheric xCO2 was measured at the atmospheric ICOS site. The sample air was drawn from the tower (56 m) to the ground level where it was analyzed using cavity ring-down spectroscopy (Picarro G2401). The data were logged as 1 min average values. Three standard gases made by FMI were used for the reference measurement. Differences between the target and measured values of these gases were within ±0.20 ppm (Kilkki et al., 2015).
2.4 Calculated data
2.4.1 pCO2 temperature correction
To correct for the temperature difference between in situ and equilibrator temperature, we took the effect of the temperature change on pCO2 into account by using the CO2SYS MATLAB program (van Heuven et al., 2011). This correction requires knowledge of another carbon system component, which is total alkalinity (from salinity) in our case. The widely used temperature correction of pCO2 suggested by Takahashi et al. (1993) is not applicable for the brackish conditions of the Baltic Sea (e.g., Schneider and Müller, 2018). The difference in temperature oscillates within ±2.0 ∘C.
2.4.2 Determination of the mixed layer depth
The mixed layer depth (zmix) was determined from the vertical temperature profiles of the CTD casts. Even though the data by the thermistor chain have higher temporal resolution than the CTD castings, they are not applied for the assessment of the mixed layer depth because they have significantly lower vertical spatial resolution. The water depth at the location of CTD casts is approximately 90 m, which is significantly deeper than the depth at the inlet location. If the mixed layer depth was deeper than the depth of 23 m at the inlet location, the water column at the inlet location was considered fully mixed. The thermocline depth, i.e., the depth of the strongest temperature gradient in the profile, was considered to represent zmix. For each CTD cast, a thermocline depth was estimated. The thermocline depths with a questionably small (<0.2 ∘C m−1) temperature gradient were discarded.
Due to the marked horizontal distance between the inlet and CTD profiling, the applicability was assessed by comparing these CTD measurements to the Pt-100 thermistor chain measurements near the inlet, which confirmed the relatively good match of the measurements with the root mean square difference of 0.6 ∘C. The CTD measurements reproduced well the hydrography of the upper water column at the inlet location, as the root mean square differences between the sites for the depths of 3, 8, and 13 m were 0.42, 0.41, and 0.25 ∘C, respectively. The temperatures at 20 m, however, showed larger difference as the root mean square error (RMSE) was 1.08 ∘C for this depth. This implies that the mixed layer depths were well reproduced using the CTD castings unless the thermocline was located close to the bottom of the inlet location.
2.4.3 Estimation of Fas
The estimation of the exchange of CO2 between the sea and atmosphere used in this study is based on two methods: (1) the eddy covariance method, using the data gathered using a micrometeorological flux tower erected on the western shore of the island and (2) a wind-speed-based flux parameterization. Due to strict quality control, the eddy covariance method was applicable for only 18 % of time, and for the rest of the time, the parameterization was used.
Both methods have pros and cons, due to which they complement each other. The eddy covariance method considers the integrated flux within a large footprint area, whereas the parameterization is based on the pCO2 measurement at a single point at the depth of 4.5 m. The large footprint area may contain spatially heterogeneity in seawater pCO2. In some cases, the measurement at the depth of 4.5 m may not represent the surface conditions. Additionally, the parameterization of gas transfer velocity is based on the wind speed, which does not contain all the information about the surface turbulence used alone, in particular close to land masses.
The eddy covariance fluxes for the air–sea exchange of CO2 were calculated at 30 min intervals. This flux measurement is based on the closed-path non-dispersive infrared gas analyzer (LI-7000, LI-COR). The sample air tubing has a 30 cm Nafion drier (PD-100T-12-MKA, Perma Pure) in order to eliminate the water vapor interference of CO2 fluxes. The covariance of 10 Hz vertical wind velocity (w) and CO2 molar fraction (xCO2) data was calculated for each 30 min averaging period. These fluxes were corrected for the high-frequency attenuation by using a transfer function that was calculated from the deviation of the normalized w–CO2 cospectrum from the cospectrum of sensible heat flux. Only stationary CO2 flux conditions were included because during non-stationary conditions, the measured fluxes do not represent the exchange between the surface and the atmosphere. Only westerly winds were considered (180–330∘) here as the flux footprint during these cases originates from the sea. A small amount of flux data was excluded from the analysis because the reference gas pipeline for the CO2 analyzer was leaking. More information about the flux system and its quality control can be found in Honkanen et al. (2018).
We used an air–sea exchange estimation based on the quadratic relationship created by Wanninkhof (2014) for the times without valid eddy flux measurements (82 % of the time). Wind speed was measured with the micrometeorological flux tower on the western shore, and data were converted to wind speed at the height of 10 m, U10. As the wind speed is not precisely measured at the height of 10 m, we corrected wind speed assuming a logarithmic wind profile and a constant surface roughness of 0.5 mm, an average value that is based on the data of Honkanen et al. (2018). More details about the compatibility of the parameterization for this specific site can be found in Appendix A1.
2.4.4 Alkalinity–salinity relationship
We use total alkalinity as a second carbon system variable in our calculations. The total alkalinity used here is calculated using the alkalinity–salinity relationship:
where salinity is unitless and total alkalinity has the unit of µmol kg−1. This is based on the samples gathered from the flow-through system at Utö in summer 2017 (Lehto, 2019). Total alkalinity was determined from these samples by using the potentiometric titration method (Metrohm Titrino 716). The samples were conserved with mercury chloride before the analysis in Finnish Environment Institute's research laboratory in Helsinki. The titrant and the rinsing water had a salinity of 7. Alkalinity was calculated from the titration curve based on the least squares method. More information on the alkalinity–salinity relationship can be found in Appendix C.
2.4.5 The calculation of the pCO2 changes generated by different processes
The surface pCO2 is affected by processes that change the concentrations of dissolved inorganic carbon (DIC) or total alkalinity (TA), or through changes in temperature, salinity, or pressure affecting the carbonate system balance (Takahashi et al., 1993). In contrast to pCO2, DIC and TA behave conservatively with respect to temperature changes and mixing of water masses, when expressed in concentration units of µmol kg−1 of seawater.
As DIC (see Appendix B) is introduced to or removed from the dissolved inorganic pool, its change is depicted by the so-called Revelle factor, Re (Sarmiento and Gruber, 2004):
DIC in surface water is affected by the CO2 exchange with the atmosphere, biological transformations, precipitation/dissolution of calcium carbonate, freshwater input, and the mixing of water masses. The processes controlling the freshwater balance include evaporation, precipitation, and the formation and melting of sea ice. Precipitated water or melted sea ice may produce a layer of low salinity water at the sea surface, which in most cases is likely to be eroded easily by turbulence.
Biological processes affecting pCO2 include all transformations between the inorganic and organic carbon pools, i.e., photosynthesis and respiration. The mixing processes include horizontal advection, vertical diffusion, and vertical entrainment.
TA (see Appendix C) is mainly altered by the formation and dissolution of calcium carbonate. A smaller contribution to TA originates from nitrogen transformations through biological processes and the mixing processes. TA is not affected by the air–sea exchange of CO2. The effect of calcifying primary producers in the carbon pool can be neglected for the open Baltic Sea (Tyrrell et al., 2008). However, calcifiers may have an effect on the carbon cycle in the benthic zone.
Temperature affects the dissociation constants and solubility of gases, which further alters the CO2 partial pressure. For stable oceanic conditions, this change is well documented (Takahashi et al., 1993), but in estuary conditions, the temperature effect on pCO2 varies significantly (Schneider and Müller, 2018). Based on the choice of the parameterization of dissociation constants, this value might show small variations as a function of temperature and salinity (Orr et al., 2015). Similarly to temperature, salinity and pressure also affect the dissociation constants.
In this study, we investigate the contribution of individual processes and drivers to the diurnal variation of pCO2. We are considering the pCO2 changes that are generated by the changes in DIC or by temperature fluctuations. DIC changes are further divided into the changes that are caused by the air–sea exchange of CO2 or by biological transformations. There are multiple other processes that have the potential to affect the pCO2 that are not included in the analysis. See Appendix C1 for more information on the omitted processes.
Calculations of the carbon system were performed using the CO2SYS MATLAB program (van Heuven et al., 2011). Dissociation constants K1 and K2 were calculated based on the work of Millero (2010), and the sulfate contribution is based on the work of Dickson et al. (2007). We implemented the total boron parameterization of Kuliński et al. (2018), which is based on the empirical data of the Baltic Sea, in CO2SYS.
First, the carbon chemistry is calculated in CO2SYS for each hour based on the measured partial pressure of CO2, parameterized total alkalinity (see above), temperature, and salinity. This results in hourly data of DIC at the sea surface.
In the case of the hourly temperature-related pCO2 change, we assume that DIC and TA do not change. Using the temperature of the next hour together with the previously known DIC and TA, we calculate the new pCO2 in CO2SYS that is governed solely by the temperature change.
In the case of air–sea exchange and biological transformations, we calculated how much DIC has changed over 1 h by these processes separately and add this DIC change, dDIC, to the original DIC content. Then, we calculated the carbon system using this new DIC and the unaltered total alkalinity in order to get the new pCO2.
We assume that the new inorganic carbon (dDICA) derived from the air–sea exchange of carbon dioxide is evenly distributed within the mixed layer. The DIC change due to the air–sea exchange of CO2 is calculated as
where δt is time change, in our case 1 h. The value of Fas is calculated using either the eddy covariance method or the wind-speed-based parameterization, with the former given priority when passing our rigorous quality control procedure (18 % of the time considered in this study).
We inferred the biological effect on DIC indirectly from the oxygen measurements by assuming the Redfield ratio (Redfield et al., 1963). As inorganic carbon is consumed (or released), a corresponding amount of oxygen is released (or consumed):
The ratio of 106 C : −138 O refers to the Redfield ratio of carbon to oxygen (Redfield et al., 1963). However, this ratio is based on average oceanic conditions and may show variations in space and time. The last term in the equation takes the effect of air–sea exchange of oxygen into account. This flux, FO2, is calculated similarly to the carbon dioxide flux (Eq. A1) by using the gas transfer velocity and the oxygen solubility, the measured oxygen concentration in seawater, and the oxygen concentration calculated for hypothetical equilibrium with the atmosphere. Oxygen solubility was calculated according to the salinity–temperature dependence fit of Garcia and Gordon (1992), which is originally based on the work of Benson and Krause (1980). The Schmidt number of oxygen and gas transfer velocity were calculated according to Wanninkhof (2014). Oxygen concentrations can also change due to mixing, the contribution of which remains unknown.
For each day, the cumulative sums of the hourly pCO2 changes generated by a specific process (temperature, biological transformations, or air–sea exchange of CO2) were calculated for 00:00–23:59 UTC, in order to know how the specific process alters the pCO2 during a day. Finally, the mean of the cumulative sum was removed from these values, because we are interested in the daily changes, not the absolute values. pCO2,i′ is the cumulative pCO2 change between the ith and the first hour:
where i is the index of each hour and the angle brackets denote the averaging.
In addition to the pCO2 evolution generated by the air–sea exchange of CO2, biological transformations, and temperature alone, we also examined the pCO2 evolution generated by these three processes simultaneously. This is calculated using the DIC that is altered by both the air–sea exchange of CO2 and biological transformation, and additionally taking into account the temperature change. However, this pCO2 change is only used for the verification of the method and as a base for the discussion of the shortcomings and potential improvements.
Throughout the results, we use the range, r, to describe the diurnal pCO2 variability. The range, or the peak-to-peak amplitude, is defined as a difference between the diurnal pCO2 maximum and minimum.
3.1 Environmental conditions and seasonal pCO2 variability
Our observations start in July 2018 during the so-called blue water period (Schneider and Müller, 2018), a phase in early summer that is characterized by close-to-zero net community production between the spring and the midsummer bloom events (Andersson et al., 2017). As it is typical for this period, chlorophyll a concentration was low, which is reflected in a low relative fluorescence unit (Fig. 2c). At the same time, surface pCO2 was close to equilibrium with the atmosphere. In mid-July, a cyanobacteria bloom developed, as is typical for the study area and time of the year (Kraft et al., 2021). The primary production activity lowered the pCO2 below 200 µatm. This low pCO2 level persisted for about 1 month (Fig. 3a). The measured oxygen concentration and calculated equilibrium concentration were close to equilibrium in the beginning of July, but due the cyanobacteria bloom, the oxygen concentrations diverged, and for a week, the sea was strongly supersaturated. After the pCO2 had increase to almost 600 µatm, another bloom occurred in early September and caused a second pCO2 minimum. After this bloom, the measured oxygen stayed higher than the equilibrium concentration for a week. In late September 2018, pCO2 peaked at 800 µatm. This is a result of the deepening of the mixed layer depth (Fig. 2a), which causes vertical entrainment of sub-thermocline water masses that are enriched in DIC due to the remineralization of organic matter. During wintertime, the pCO2 slowly decreased and reached equilibrium with the atmosphere by the end of March. Also, thorough the winter, the sea was mostly a sink of oxygen and the measured oxygen concentration predominantly increased. Chlorophyll a fluorescence peaked again in April 2019 as a result of the spring bloom. Simultaneously, the pCO2 dropped to 200 µatm, where it stayed for 2 months. The measured oxygen peaked at 475 µmol at the end of April, and the sea was supersaturated with oxygen for over 2 months.
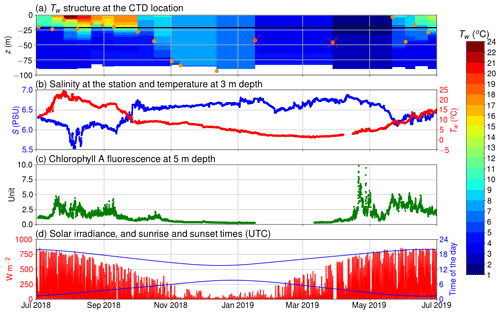
Figure 2(a) The temperature of the seawater (Tw) assessed by the CTD casts and the depth of thermocline (orange circles); discarded thermocline depths are marked with a red cross; the horizontal black line depicts the depth of the inlet; (b) salinity at 5 m depth and temperature at 3 m depth; (c) chlorophyll a relative fluorescence at 5 m depth; (d) solar irradiance (in red) and sunrise and sunset times (in blue) in UTC.
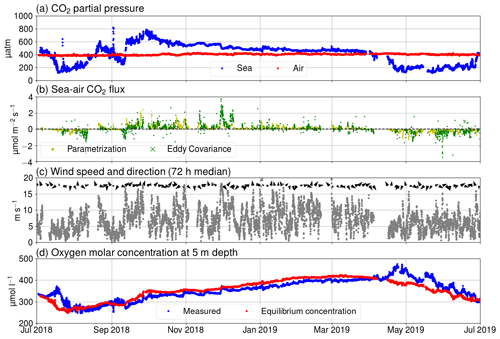
Figure 3(a) pCO2 in air (red) and seawater (blue), (b) Fas measured using the eddy covariance method (green) and calculated using Eq. (A3) (yellow), (c) wind speed (gray dots) and direction (black arrows), and (d) oxygen molar concentration of seawater as measured (blue) and calculated for hypothetical equilibrium with the atmosphere (red).
Over the course of the year studied, the sea was a sink of atmospheric carbon for approximately 4 months. Generally, the seasonality of surface pCO2 at Utö is similar to the open pelagic conditions in the Baltic Proper (Wesslander et al., 2010; Schneider and Müller, 2018) but the maximum value (800 µatm) in autumn is considerably higher than observed in the Baltic Proper (600 µatm). This could be due to the fact that the water depth at the sampling location is low, and thus remineralized CO2 from the sediment surface can be directly entrained into surface waters upon vertical mixing.
The thermocline was located at the depth of 20 m during most of the time in summer 2018. In autumn, the thermocline deepened, and in winter the water column was considered to be completely mixed. The thermocline may have only been shallower than the inlet depth of the seawater supply occasionally, e.g., in spring 2019, when a shallow thermocline formed for a short period. Therefore, most of the time, our flow-through setup was supplied with water from the mixed layer. We did not observe surface freshwater layers or permanent ice coverage during the measurement period that would be of relevance for the interpretation of our findings.
3.2 Examples of diurnal pCO2 variability
Two contrasting examples of the diurnal pCO2 variability at the beginning of September and in late December 2018 are shown in Fig. 4. On 3 September 2018, we observed a large diurnal pCO2 range (maximum–minimum) of 108 µatm. The oxygen-derived biological pCO2 signal shows a very similar pattern, indicating that this large pCO2 diurnal variability is mainly a result of biological transformations. Minor deviations between observed pCO2 and the biologically driven changes based on oxygen dynamics occur early in the morning and late in the evening. The air–sea exchange had a negligible effect on the pCO2 on that day, because the pCO2 difference between the sea and atmosphere was close to zero. Including temperature as a driver into our model of the surface pCO2 variability slightly increases the deviation from the observed hourly changes. It is possible that this is due to a too-low oxygen-derived biological component. In Sect. 3.2.5, we give evidence of a slightly too-small biological component in September.
The pCO2 on 20 December 2018 was decreasing almost linearly. This example shows that the oxygen-derived pCO2 variation is higher than the observed pCO2 variation in winter. The oxygen is primarily altered by mixing and air–sea exchange of oxygen. This issue is discussed in Sect. 3.2.5. Both the air–sea exchange of carbon and gradual cooling of the water contribute to the decrease of surface pCO2.
The largest daily pCO2 range (503 µatm) was detected on 22 July. This extreme case can be attributed to an upwelling event as the water at the marine station, measured by the thermosalinograph, cooled by 5 ∘C simultaneously. Most of the cooling effect did not reach the thermistor at 3 m, as the temperature at the thermistor chain cooled less than 2 ∘C at 3 m depth. Observations made during this upwelling event were discarded from the following analysis of the diurnal pCO2 variability. Another large pCO2 change (452 µatm) occurred on 2 September, but the water temperature at the station changed approximately 1 ∘C, and thus we did not exclude the data from this day from our analysis.
3.2.1 Observed diurnal pCO2 variability
The observed diurnal variability of pCO2 was lowest during the wintertime (Fig. 5). On average, the monthly median range (maximum–minimum) in November–February was only 4 µatm. Within the winter months, February revealed the lowest monthly median range and the lowest range between the 10th and 90th percentiles: less than 11 µatm daily variation was observed for 80 % of the time. In wintertime, no clear diurnal pattern is visible, which goes along with varying times for the daily minimum and maximum pCO2. This absence of a diurnal pattern in pCO2 during winter is consistent with the findings of Lansø et al. (2017) for the Baltic Proper.
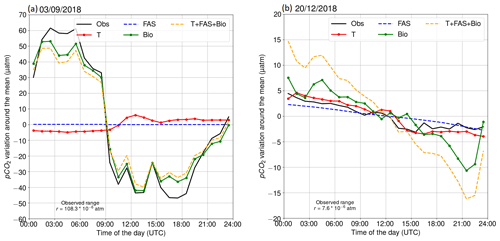
Figure 4Diurnal pCO2 variability on (a) 3 September 2018 and (b) 20 December 2018. The black line is the observed (Obs) evolution. Other lines represent the calculated pCO2 evolution, driven by different processes: red indicates temperature (T), blue indicates the air–sea exchange of carbon dioxide (FAS), green indicates biological transformations (Bio), and orange indicates the combined effect of all processes (T + FAS + Bio).
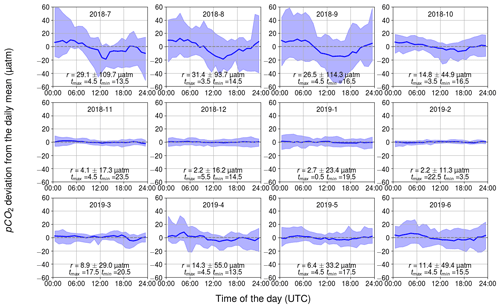
Figure 5Observed diurnal pCO2 variability. Displayed are the hourly binned median values (line) and the range between the minimum of the 10th percentile and the maximum of 90th percentile (ribbon). The y axis shows the pCO2 deviation (in µatm) and the x axis shows the hour of the day. The mean and standard deviation of the daily range, r, and the timing for the maximum and minimum pCO2 are also given.
In April, the observed diurnal pCO2 variability starts to show a sinusoidal form, which remains until October. The diurnal pCO2 minimum occurs during the afternoon and the maximum in early morning. At approximately 09:00 UTC (12:00 LST – local summer time), the pCO2 is closest to the diurnal mean. The monthly median range of pCO2 increased until August, which had the highest monthly median range of 31 µatm. In the Baltic Proper, the highest diurnal pCO2 variability (27 µatm) was observed in September (Lansø et al., 2017). However, this difference is likely due to the interannual variability as different years are compared. There is large variability in diurnal pCO2 over the course of a single month during the productive season. During this time, a single day may deviate significantly from the monthly median value. According to the 10th and 90th percentiles, 80 % of the days in September occur within a large range of 114 µatm.
3.2.2 Biology-related diurnal pCO2 variability
The diurnal pCO2 variability induced by biological activity and inferred from changes in the oxygen concentration are closely similar to the observed pCO2 dynamics (see Figs. 4–6). In both cases, sinusoidal diurnal variability with the maximum in the morning and the minimum in the afternoon during April–September is observed and the monthly median ranges are of similar strength. During nighttime, respiration (both heterotrophic and autotrophic) prevails, which increases DIC and thus also pCO2. Solar irradiance intensifies as the day progresses and the carbon fixation outweighs the respiration, causing DIC to decrease. For our shallow sampling location, it is further possible that benthic processes impact surface water carbon dynamics, especially when the water body is completely mixed.
In summer, the daytime increase in temperature partly counterbalances the pCO2 reduction caused by primary production. The temperature-driven diurnal pCO2 maximum and the biologically controlled pCO2 minimum occur at approximately the same time in the afternoon. However, the temperature effect is significantly smaller than the impact of primary production.
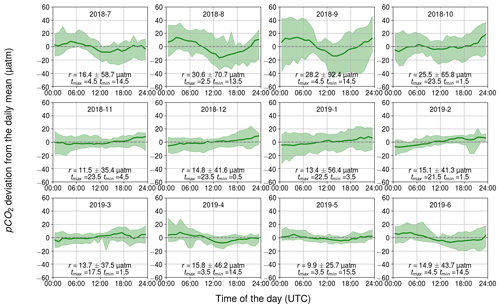
Figure 6Observed monthly pCO2 diurnal variability generated by biological transformations, showing the binned median and difference of the minimum of the 10th percentile and the maximum of the 90th percentile. The y axis shows the pCO2 deviation (in µatm) and the x axis shows the hour of the day. Range, r, and the time for the maximum and minimum pCO2 are also given.
The largest observed and modeled biological pCO2 diurnal variability occurs in August and is twice as large as the range observed during the spring bloom. On the one hand, the temperature is at its annual maximum during July–August, which favors phytoplankton growth (Trombetta et al., 2019), but on the other hand, the solar irradiance is already decreasing from its annual maximum during June–July. During the spring bloom, chlorophyll a fluorescence was high compared with the one during August, when the highest pCO2 variation is observed. However, microbial respiration tends to increase towards higher temperatures (Lopez-Urrutia et al., 2006), and thus the highest respiration rates are expected during July–August, contributing to the large amplitude of the diurnal cycle. It is possible that in spring, the daily pCO2 range is lower than in autumn due to the deeper mixed layer in spring (Fig. 2a) causing the production to be distributed across a larger water volume.
Our data set suggests that, on average, the biological component controls pCO2 diurnal variability, but on specific days during the biological season, other components (especially mixing) can have a stronger impact, as Wesslander et al. (2011) have shown.
During winter, the diurnal pCO2 pattern generated by the biological processes revealed a positive trend over the course of a day, which could indicate the remineralization of organic matter. The fact that this directional trend is not seen in the observed pCO2 could be due to the CO2 release to the atmosphere counterbalancing the biological effect. However, it is implausible that the remineralization occurs for the entire winter and is even strongest in February.
3.2.3 Temperature-related diurnal pCO2 variability
The daily variation in seawater temperature follows the cycle of solar irradiation. The highest monthly average of daily temperature range (daily maximum temperature–daily minimum temperature) was in July with 1.6 ∘C and the lowest in February with 0.2 ∘C.
The diurnal pCO2 variability driven by changes in temperature is generally small (Fig. 7). Apart from June, July, and August, the monthly median range was 3 µatm or less. The largest monthly median range occurred in July (5 µatm), when the solar irradiance reaches its annual maximum (Fig. 2d). Still, for 20 % of the days in July, a temperature-related diurnal variability of pCO2>27 µatm was observed.
During months with high solar radiation, i.e., March–September (Fig. 2d), the maximum of the temperature-related diurnal pCO2 cycle occurs at noon and the minimum in the middle of the night or in the early morning. In winter, the temperature-related pCO2 changes do not show a clear diurnal pattern nor directional trend.
The measurement depth of the temperature is 3 m. Directly at the sea surface, we would expect higher temperature-induced pCO2 variability since solar irradiance decreases with depth.
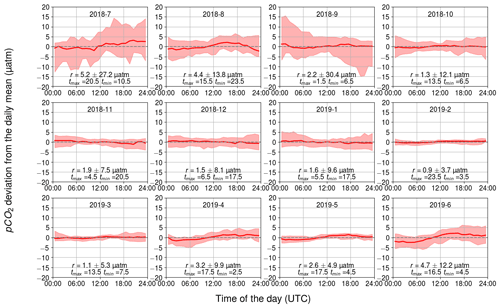
Figure 7Temperature-induced cumulative daily changes in pCO2, shown as the monthly climatological median and the difference of the minimum of the 10th percentile and the maximum of the 90th percentile. The y axis shows the pCO2 deviation (in µatm) and the x axis shows the hour of the day. Range, r, and the time for the maximum and minimum pCO2 are also given.
3.2.4 Diurnal pCO2 variability generated by the air–sea CO2 flux
Diurnal pCO2 fluctuations generated by the air–sea exchange of CO2 exhibit a clear trend-like pattern (Fig. 8) due to the nature of the process. The direction of the air–sea CO2 flux is controlled by the sign of the CO2 partial pressure difference between the sea surface and the atmosphere. As the atmospheric pCO2 is relatively stable compared to that of the sea, the flux direction is largely controlled by the seawater pCO2. The trend in the diurnal pattern of pCO2 generated by air–sea exchange thus represents the net carbon uptake of the Baltic Sea in summer when the sea surface pCO2 is lower than atmospheric pCO2, and vice versa in winter.
The magnitude of the air–sea fluxes is largest during September–October when a large partial pressure gradient and high wind speeds co-occur. In these months, the monthly median range was 10 µatm or higher. In contrast, the effect of air–sea exchange on diurnal pCO2 variability is almost negligible (less than 2 µatm) when the sea and atmosphere were nearly balanced with respect to pCO2, as during December–March, or when the wind speeds are low, as in the summer months.
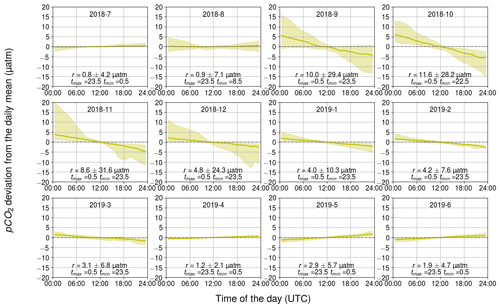
Figure 8Monthly pCO2 diurnal variability generated by the air–sea exchange of carbon dioxide, showing the binned median and difference of the minimum of the 10th percentile and the maximum of the 90th percentile. The y axis shows the pCO2 deviation (in µatm) and the x axis shows the hour of the day. Range, r, and the time for the maximum and minimum pCO2 are also given.
3.2.5 Comparing observed and estimated pCO2 variability
When comparing the observed hourly change in pCO2 and the calculated change that takes into account the three processes – air–sea exchange, biology, and temperature (Fig. 9) – we found that the overall RMSE between all hourly modeled and observed pCO2 changes was 10 µatm. RMSE was 9–14 µatm during July–October, while it was less than 3–6 µatm during the other seasons. The scatter in Fig. 9 is visibly highest during July–October. These months showed the highest observed diurnal pCO2 variability, which may have a direct effect on the increased error. For each month, we divided the RMSE value with the average absolute change in hourly pCO2 and found this ratio to be 1.26 on average during March–October, whereas during November–February it was 3.29 on average. Thus, the error introduced by the model during these winter months, though comparatively small in its absolute value, is large compared to the observed variability, which suggests that the estimates of the biological component during the wintertime should be interpreted with care. This, however, does not have a significant effect on the analysis, since the biological activity in winter is negligible (see Fig. 2c).
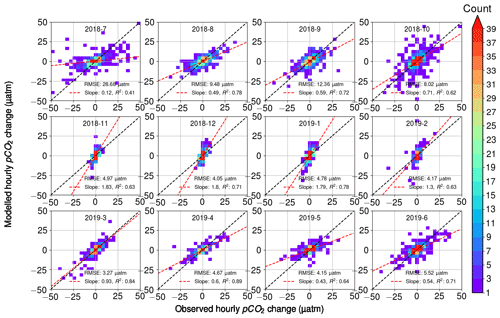
Figure 9Modeled hourly pCO2 changes as a function of observed pCO2 changes. Color indicates the number of observations within bins of 2 µatm width. For each month, the RMSE between the model and the observations is given, as well as the slope of the best fit (red line) with its correlation coefficient. The black line is the identity (1:1) line.
The fitted slope between the modeled and observed hourly pCO2 changes appears to vary during the seasons. During the early winter months (November–January), the modeled pCO2 changes are twice as large as the observations (see the slope of 2.1). During the late winter (February–March), the model and observations give the closest match with the slopes of 1.0–1.3. From April to October, the slope varied between 0.3 and 0.7, with the smallest slopes in July (0.3) and May (0.4).
Most of the variation in the modeled pCO2 originates from the oxygen-derived biological processes, and thus we argue that the different slopes in observations and modeled data are related to the parameterization of the biological processes. To identify the reason for the mismatch between model and observations, we performed a similar analysis to that in Fig. 9 but separately disabled the oxygen flux between the atmosphere and sea (i.e., assuming all oxygen changes to originate from the biological transformations), as well as temperature-induced pCO2 changes and air–sea CO2 flux. However, these modifications of our pCO2 model proved to only have a negligible effect on the slopes. Possible remaining sources of error thus include the parameterization of the air–sea exchange of oxygen, the parameterization of the mixed layer depth, and the carbon–oxygen ratio in Eq. (4).
It is possible that the seasonal slope changes in Fig. 9 are due to the fact that the oxygen concentration changes are not well-constrained by the O2 flux. This could be due to a time lag between the O2 flux at the air–sea interface and the O2 concentration change at 5 m depth. It is indeed likely that the wind speed parameterization of O2 flux provides a good estimate of the O2 air–sea flux, but that the flux at the surface is challenging to translate into the O2 concentration changes at 5 m depth at 1 h resolution. In summer, the oxygen flux is directed from the sea to atmosphere, and thus its effect on the biological component during daytime should be positive. If this process is not taken into account, we might end up with an underestimated biological component, i.e., low slopes in Fig. 9. In winter, vice versa would happen.
A bias in our estimation of the mixed layer depth may also introduce an error in the modeled pCO2 change. It is possible that in spring, the vertical redistribution of surface O2 fluxes may not extend to the mixed layer depth. This would cause the gas exchange term of oxygen to be underestimated in Eq. (4), leading to the biological pCO2 component in the model to be too low. In autumn, the calculated mixed layer depth might be too shallow to fully capture the vertical mixing of surface O2 fluxes. A major limitation in this regard is our definition of the mixed layer depth as the water depth at the sampling location in cases when the true mixed layer depth at the CTD location was found deeper than the water depth in the inlet location. This limitation is critical, because it would not capture the loss of O2 due to lateral mixing with deeper waters close to the sampling location. This would cause the gas exchange to be overestimated and the biological pCO2 component to be too high.
The Redfield ratio for CO2–O2 (−0.77) used in this study is based on an oceanic average (Redfield et al., 1963). To explain, the slopes between the model and the observations (−0.3 to −2.1) would require a CO2–O2 ratio of −0.37 in winter and as high as −2.5 in some summer months. The CO2–O2 ratio of respiration (the respiratory quotient) depends on the organic substrate in question, the degree of its oxidation, and the metabolic pathway used. This quotient may indeed vary between −0.13 and −4.00 (Robinson, 2019). In contrast, the required photosynthetic quotient of −2.5 in July appears very high compared with typical values (Laws, 1991). Wesslander et al. (2011), for example, determined the CO2–O2 ratio in April 2006 in the Baltic Proper to be −1.0, with some diurnal variation. We thus conclude that the changes in respiratory and photosynthetic quotients alone cannot explain the seasonality in the slopes.
3.3 Effects on the air–sea exchange of CO2
The diurnal pCO2 variability can have a significant effect on the instantaneous air–sea CO2 fluxes. The sign of the integrated daily air–sea CO2 flux can even change when the pCO2 values at the sea surface and in the atmosphere are close to equilibrium, as was observed on 22 July and 2 September (data not shown).
The largest observed monthly median ranges in pCO2 occurred during July–September (27–31 µatm). During this time, the pCO2 varied from slightly above 100 µatm to 800 µatm. In addition to the wind speed, the pCO2 difference between the sea and the atmosphere controls the air–sea flux. The greatest relative effect on the daily flux occurs when the sea pCO2 varies close to the atmospheric pCO2, i.e., at approximately 400 µatm. In late July and early August 2018, the sea was a sink, and in late August and September, the sea was a source of CO2 to the atmosphere at the study site. The diurnal pCO2 variability during these months is similar, with a maximum before noon and a minimum in the afternoon. However, in late July and early August, the pCO2 difference between the sea and atmosphere is smallest before noon and largest in the afternoon, whereas in late August and September, the situation is reversed: the largest difference is before noon and the smallest is in the afternoon.
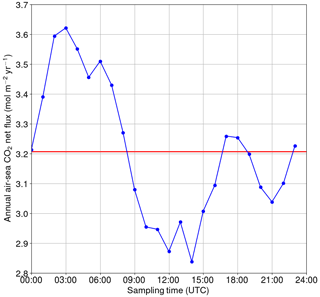
Figure 10Annual net flux of carbon dioxide between the sea and atmosphere if only one measurement per day is used. The reference (the red line) is based on high-frequency data.
The discussion above only takes into account the diurnal variability of the air–sea pCO2 gradient even though the flux also depends on the gas transfer velocity. This might also exhibit diurnal cyclicity, especially during clear skies in the coastal regions, where spatially uneven heating of the ground generates pressure gradients and thus winds. The most popular parameterizations for gas transfer velocity are either quadratic or cubic functions of the wind speed and thus even small changes in wind speed have a large impact on the flux.
For the hypothetical case of a single sampling event per day, we calculated how the annual net exchange of carbon dioxide between the sea and atmosphere would vary depending on the sampling time (Fig. 10). The calculations were performed using the flux parameterization of Wanninkhof (2014). The reference net exchange (red line in Fig. 10, i.e., the “true” value) is calculated using the high-frequency 1-hourly data, whereas the other fluxes are calculated using only one measurement at the daytime indicated on the x axis. The closest match with the “true” net flux is achieved when sampling the seawater at 00:00, 08:00–09:00, 16:00–19:00 or 23:00 UTC. In contrast, sampling between 00:00 and 09:00 UTC causes an overestimation of the net flux by up to 12 %, whereas sampling between 09:00 and 18:00 UTC leads to an underestimation of up to −12 %. The sinusoidal shape of the net flux bias as a function of the sampling time clearly originates from the biological component of surface pCO2, but the deviation from the sinusoid around 15:00–20:00 UTC must originate from the turbulence parameterization (wind speed) as such a shape is not observed in the pCO2.
The diurnal variability of sea surface pCO2 and the contributions of its drivers were studied at the Utö station in the Archipelago Sea of the Baltic Sea. Multiple processes affecting the diurnal pCO2 variability at Utö were distinguished and their interplay was found to depend on season, similarly to what was previously shown for the east of Gotland by Wesslander et al. (2011). At Utö, the largest variability was found during July–September, when the monthly median of the diurnal pCO2 varied in the range of 27–31 µatm. This pCO2 variability was mostly generated by the biological transformations (i.e., the production and respiration of organic matter). However, individual days showed significantly higher variations. Extreme pCO2 variations exceeded 500 µatm a day and were attributed to upwelling of CO2-enriched water masses. Diurnal pCO2 variability was less pronounced in wintertime, which is comparable to the observations in the Baltic Proper (Lansø et al., 2017). Thus, on average, the magnitude and the timing of the diurnal pCO2 variability at Utö are similar to the ones of the pelagic conditions in the Baltic Proper, except for coastal upwelling at the study site.
Assessment of the annual air–sea flux based on the entire data set or individual 1 h sampling times revealed a potential bias caused by the time of sampling of up to 12 %. This finding suggests that data from moving platforms which do not resolve the diurnal cycle, like RVs or SOOP lines, can lead to substantial biases in flux calculations or the estimation of natural variability.
These findings emphasize the importance of continuous measurements at fixed locations providing a high temporal resolution, in order to complement SOOP-based observations that achieve high spatial coverage. Our autonomous high-frequency measurements of the seawater carbonate system at a fixed site have proven to be valuable in the assessment of the short-term variability of the carbonate system. However, as European seas are spatially highly heterogeneous, our findings call for organized efforts to map the diurnal variability of the carbon system.
The CO2 exchange between the atmosphere and the sea, Fas, is driven by the difference in CO2 partial pressure () between the surface seawater and atmosphere, or more precisely, the differences in fugacity, which refers to the effective partial pressure of CO2 that takes into account the non-ideal gas behavior of CO2. CO2 partial pressure and fugacity only differ slightly and, for this reason, only partial pressure is used from now on. The efficiency of the exchange through the diffusive boundary layers of the gas and liquid fluids is defined by the gas transfer velocity, k. Thus, Fas may be written as
where K0 is the solubility of CO2.
The effects of the kinematic viscosity of seawater and the diffusion efficiency of CO2 on k are taken into account by including the ratio of momentum diffusivity in mass diffusivity, the Schmidt number (Sc), in k:
Since the Schmidt number is a function of temperature, it is normalized with the Sc of seawater at 20 ∘C, a value of 660. A wind speed measured at 10 m (U10) is most commonly used to parameterize k660, and probably the most well-known parameterization is a quadratic relationship proposed by Wanninkhof (1992), which was revised by Wanninkhof (2014):
A1 The parameterization of gas transfer velocity
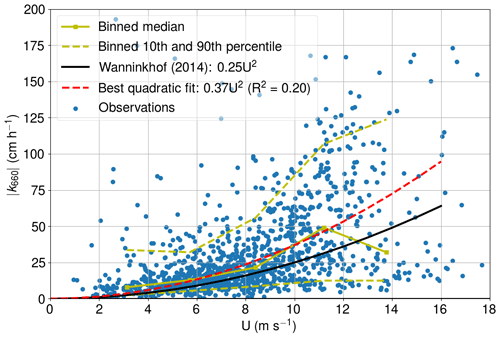
We patched the CO2 air–sea flux time series using the U10-based parameterization for k660 proposed by Wanninkhof (2014). The applicability of this parameterization for the western marine region of Utö was assessed by calculating the absolute value of k660 (Fig. A1) from the measured CO2 air–sea flux (from eddy covariance), partial pressure difference, solubility (Weiss, 1974), and the Schmidt number (Wanninkhof, 1992). Only cases with southwestern (180–330∘) winds and strong pCO2 difference (>30 µatm) were considered. CO2 flux outliers were discarded so that we only included the fluxes that are within 2 standard deviations from the median.
Non-stationarity is one of the determinant factors for the quality of direct flux measurement, and thus non-stationary fluxes are discarded. Here, this means that the mean of 5 min fluxes can deviate less than 30 % from the 30 min flux. The fully stationary condition is purely a theoretical concept, and the threshold for the accepted deviation from this is a matter of choice.
The best quadratic fit (0.37 ) is somewhat larger than the parameterization proposed by Wanninkhof (2014), which might indicate enhanced gas transfer due to the coastal characteristics of the study site. However, for the comparability, we stick with the common parameterization by Wanninkhof (2014). Low and medium wind speeds are well packed, whereas the 10th and 90th percentiles move further away from each other at high wind speeds. The parameterization of Wanninkhof (2014) shows the highest deviation from the binned median values at highest wind speeds. The binned median at the highest wind speeds is low compared with the results of Wanninkhof (2014), which may indicate fetch limitation. More observations at high wind speeds are thus required for the in-depth analysis.
Gaseous CO2 dissolves into water, where part of it hydrates into carbonic acid (H2CO3). Dissolved CO2 and carbonic acid are not easily distinguished, and thus the sum of their concentrations is denoted as [CO2*]:
Henry's law describes the relationship between the fugacity of gaseous CO2, which is in equilibrium with the underlying water, and the dissolved concentration of CO2:
Carbonic acid dissociates to hydrogen ion and hydrogen carbonate (also known as bicarbonate), which further dissociates to carbonate () and hydrogen ions. The equilibrium states
Solubility and dissociation constants (K1 and K2) depend on the free energy of the reaction and thus are functions of temperature and pressure. As these stoichiometric constants are defined using concentrations instead of ion activities, they are also a function of salinity.
Dissolved carbon dioxide, carbonic acid, bicarbonate, and carbonate ions form the pool of total dissolved inorganic carbon (DIC):
DIC is a conservative quantity; i.e., it does not vary as temperature or pressure change. The concentrations of different DIC species change, but the sum of these concentrations remains the same if no carbon is added to or removed from the system.
If nutrients and photosynthetically active radiation are available, dissolved CO2 is transformed into organic matter through the process of photosynthesis. When phytoplankton and other aquatic organisms respire, the opposite occurs and CO2 is released. Through microbial degradation in water or in sediments, dissolved organic matter is transformed again into inorganic carbon.
Of all the parameters of the carbonate system, one can only measure pCO2, DIC, TA, and pH (the negative logarithm of hydrogen concentration). To gain the complete description of the carbonate system, one should know at least two of these variables in addition to the information on seawater temperature (T), salinity (S), and pressure (P). Ideally, the effect of dissolved organic matter on total alkalinity should also be known. From Henry's law (Eq. B2), we see that CO2 fugacity depends on the solubility and dissolved CO2 concentration. Both of these variables are functions of temperature, salinity, and pressure. The non-conservative behavior of [CO2*] is due to the effect of the dissociation constants, K1 and K2.
Another important variable for the carbonate system is total alkalinity (TA), which is defined as the excess of proton acceptors (acids) over donors (bases). For most practical purposes, it is sufficient to only include carbonate alkalinity, boron alkalinity, and a component from the self-dissociation of water (which is commonly referred to as practical alkalinity):
Minor TA components include organic ions, which may have a large regional impact. In the case of the Baltic Sea, the bulk of dissolved organic matter has been shown to act as a proton acceptor (Kuliński et al., 2014). Similarly to DIC, TA is a conservative quantity.
Calcium carbonate (CaCO3) is formed in a slow precipitation process by specific calcifying organisms. The precipitation and dissolution of CaCO3 affect both DIC and TA. However, in the case of the Baltic Sea, calcifying phytoplankton only exists in the areas next to the North Sea (Tyrrell et al., 2008), and thus the formation of CaCO3 can be excluded in calculations for most parts of the pelagic Baltic Sea, including our study site. On the other hand, the weathering of fluvial CaCO3 has a determinant effect on TA in the limestone-rich southern regions of the Baltic Sea (Müller et al., 2016).
We used the pair of the pCO2 and the TA in our carbonate system calculations. The TA is parameterized using the salinity, because both of these variables are affected by the conservative mixing.
The least squares fit of the relationship between the salinity and the directly measured total alkalinity (Fig. C1) had an R2 value of 0.91.
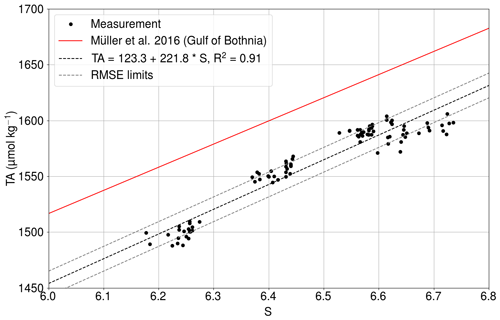
Figure C1Measured total alkalinity (black dots) as a function of salinity at Utö in 2017 (Lehto, 2019). The solid red line shows the TA–S relationship for the Gulf of Bothnia given by Müller et al. (2016), extrapolated for 2018. The dashed black line is the best fit, and dashed gray lines show the same line with the limits of RMSEs.
The RMSE between the measurements and the fit is 11.1µmol kg−1. The slope is almost identical to the dependence found for the Gulf of Bothnia by Müller et al. (2016), extrapolated for the year 2018.
C1 Processes controlling pCO2 omitted in the analysis
In our analysis to distinguish the different processes that drive pCO2 variability, we considered temperature changes, air–sea exchange of carbon, and biological transformations. Several processes were omitted.
The salinity changes are related to mixing, and thus the interpretation of the salinity effect is not straightforward and is not dealt with in this paper. The salinity effect on pCO2 is generally small: in oceanic conditions, a salinity change of 1 would generate a 9 µatm change in pCO2 (Sarmiento and Gruber, 2004). At Utö, the salinity varies less than 1.5 units during the whole year (see Fig. 2). We neglect the effect of pressure on pCO2, because we interpret surface water pCO2 at one depth.
Some of these unknown drivers, such as mixing processes and freshwater effects, are assumed to be temporally random in nature, and thus their effect on pCO2 is considered to be negligible when inspecting average diurnal cycles. Some of the processes, e.g., alkalinity-related variations affecting pCO2, are unknown and may involve diurnal cyclicity. A salinity–alkalinity relationship used in the analysis takes into account the conservative variation of these variables due to the mixing and freshwater input. Nitrogen transformations during primary production can have a small effect on alkalinity that is not considered in the salinity–alkalinity relationship.
In general, the tidal force is the most prominent process to generate a diurnal pattern on the mixing of the DIC. In this location of the Baltic Sea, the effect of the tidal currents on the water masses is very small and thus can be neglected. However, several other processes such as the upwelling can also generate mixing. The driving force of the upwelling (or downwelling) is steady wind over the sea, and at our study site, at an open sea which contains very small islands, the sea breeze cannot be completely neglected but is not expected to be strong. However, there is a possibility that the density-driven mixing has a diurnal cycle due to the diurnal heating/cooling of the surface waters.
The mixing component of the diurnal DIC variations can be large occasionally. For instance, there were clear indications of the mixing of water masses on 22 July 2018; the pCO2 varied by 503 µatm, while the water cooled by 5 ∘C. However, there are not always such clear indicators suggesting the mixing events. In order to analyze the effect of the mixing on DIC precisely, one would need to know the 3-D field of DIC and the water currents. This would require an array of carbonate system measurements. The analysis of the mixing of DIC is thus beyond the scope of this paper.
In the results and discussion section, we analyze the importance of individual drivers and the applicability of the method by comparing the calculated pCO2 changes to the observations.
The data used in this paper can be found in the Zenodo repository (https://doi.org/10.5281/zenodo.4292384, Honkanen et al., 2020).
MH, LL, JS, JDM, and GR were in charge of the conceptualization. MH performed data analysis and visualization. The manuscript was written by MH, LL, JS, JDM, and GR. SK, PY, LL, JS, and TM designed and constructed the flow-through system. TM and LL designed and constructed the flux setup. JH was in charge of data management.
The authors declare that they have no conflict of interest.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the special issue “Coastal marine infrastructure in support of monitoring, science, and policy strategies”. It is not associated with a conference.
We thank Ismo and Brita Willström for the CTD castings and maintaining the stations, and we thank Anne-Mari Lehto for providing the total alkalinity measurements. Also, thanks are due to the Integrated Carbon Observation System (ICOS) for providing the atmospheric CO2 data at Utö. We acknowledge Theo Kurten for providing guidance with chemistry and Jani Särkkä for providing guidance with mathematical formulations. The CO2SYS program and the National Land Survey of Finland are acknowledged.
We thank the referees for their insightful comments that resulted in a highly improved manuscript.
This research has been supported by the Academy of Finland (SEASINK: Evolving carbon sinks and sources in coastal seas – will ecosystem response temper or aggravate climate change?), Horizon 2020 (grant nos. JERICO-NEXT (654410) and JERICO-S3 (871153)), and The BONUS INTEGRAL. The BONUS INTEGRAL project receives funding from BONUS (Art 185), funded jointly by the EU, the German Federal Ministry of Education and Research, the Swedish Research Council Formas, the Academy of Finland, the Polish National Centre for Research and Development, and the Estonian Research Council.
The study utilized FMI and SYKE marine research infrastructure as a part of the national FINMARI RI consortium.
This paper was edited by Ingrid Puillat and reviewed by two anonymous referees.
Algesten, G., Brydsten, L., Jonsson, P., Kortelainen, P., Löfgren, S., Rahm, L., Räike, A., Sobek, S., Tranvik, L., Wikner, J., and Jansson, M.: Organic carbon budget for the Gulf of Bothnia, J. Mar. Syst., 63, 155–161, https://doi.org/10.1016/j.jmarsys.2006.06.004, 2006. a
Andersson, A., Tamminen, T., Lehtinen, S., Jürgens, K., Labrenz, M., and Viitasalo, M.: The pelagic food web, in: Biological Oceanography of the Baltic Sea, edited by: Snoeijs-Leijonmal, P., Schubert, H., and Radziejewska T., Springer, Dordrecht, https://doi.org/10.1007/978-94-007-0668-2_8, 2017. a
Andersson, A. J. and Mackenzie, F. T.: Revisiting four scientific debates in ocean acidification research, Biogeosciences, 9, 893–905, https://doi.org/10.5194/bg-9-893-2012, 2012. a
Benson, B. B. and Krause, D.: The concentration and isotopic fractionation of gases dissolved in freshwater in equilibrium with the atmosphere: 1. Oxygen, Limnol. Oceanogr., 25, 662–671, https://doi.org/10.4319/lo.1980.25.4.0662, 1980. a
Dickson, A. G., Sabine, C. L. and Christian, J. R.: Guide to best practices for ocean CO2 measurements, Special Publication 3, IOCCP Report 8, North Pacific Marine Science Organization (PICES), Sidney, British Columbia, Canada, https://doi.org/10.25607/OBP-1342, 2007. a, b
Farcy, P., Durand, D., Charria, G., Painting, S. J., Tamminen, T., Collingridge, K., Grémare, A. J., Delauney, L., and Puillat, I.: Toward a European coastal observing network to provide better answers to science and to societal challenges; The JERICO Research Infrastructure, Front. Mar. Sci., 6, 529, https://doi.org/10.3389/fmars.2019.00529, 2019. a
Feely, R., Doney, S., and S. Cooley.: Ocean acidication: present conditions and future changes in a high-CO2 world, Oceanography, 22, 36–47, 2009. a
Friedlingstein, P., Jones, M., O'Sullivan, M., Andrew, R., Hauck, J., Peters, G., Peters, W., Pongratz, J., Sitch, S., Le Quéré, C., Bakker, D., Canadell, J., Ciais, P., Jackson, R., Anthoni, P., Barbero, L., Bastos, A., Bastrikov, V., Becker, M., and Zaehle, S.: Global Carbon Budget 2019, Earth Syst. Sci. Data, 11, 1783–1838, https://doi.org/10.5194/essd-11-1783-2019, 2019. a
Garcia, H. E. and Gordon, L. I.: Oxygen solubility in seawater: Better fitting equations, Limnol. Oceanogr., 37, 1307–1312, https://doi.org/10.4319/lo.1992.37.6.1307, 1992. a
Goyet, C. and Peltzer, E. T.: Variation of CO2 partial pressure in surface seawater in the equatorial Pacific Ocean, Deep Sea Res. Pt. I, 44, 1611–1625, 1997. a
Honkanen, M., Tuovinen, J.-P., Laurila, T., Mäkelä, T., Hatakka, J., Kielosto, S., and Laakso, L.: Measuring turbulent CO2 fluxes with a closed-path gas analyzer in a marine environment, Atmos. Meas. Tech., 11, 5335–5350, https://doi.org/10.5194/amt-11-5335-2018, 2018. a, b
Honkanen, M., Müller, J. D., Seppälä, J., Rehder, G., Kielosto, S., Ylöstalo, P., Mäkelä, T., Hatakka, J., and Laakso, L.: Dataset for Diurnal cycle of the CO2 system in the coastal region of the Baltic Sea (Submitted 2020/11), Zenodo [data set], https://doi.org/10.5281/zenodo.4292384, 2020. a
Kilkki, J., Aalto, T., Hatakka, J., Portin, H., and Laurila, T.: Atmospheric CO2 observations at Finnish urban and rural sites, Boreal Environ. Res., 20, 227–242, 2015. a
Kraft, K., Seppälä, J., Hällfors, H., Suikkanen, S., Ylöstalo, P., Anglès, S., Kielosto, S., Kuosa, H., Laakso, L., Honkanen, M., Lehtinen, S., Oja, J., and Tamminen, T.: First application of IFCB high-frequency imaging-in-flow cytometry to investigate bloom-forming filamentous cyanobacteria in the Baltic Sea, Front. Mar. Sci., 8, 594144, https://doi.org/10.3389/fmars.2021.594144, 2021. a, b, c
Kuliński, K. and Pempkowiak, J.: The carbon budget of the Baltic Sea, Biogeosciences, 8, 3219–3230, https://doi.org/10.5194/bg-8-3219-2011, 2011. a, b
Kuliński, K., Schneider, B., Hammer, K., Machulik, U., and Schulz-Bull, D. E.: The influence of dissolved organic matter on the acid–base system of the Baltic Sea, J. Mar. Syst., 132, 106–115, https://doi.org/10.1016/j.jmarsys.2014.01.011, 2014. a
Kuliński, K., Szymczycha, B., Koziorowska, K., Hammer, K., and Schneider, B.: Anomaly of total boron concentration in the brackish waters of the Baltic Sea and its consequence for the CO2 system calculations, Mar. Chem., 204, 11–19, https://doi.org/10.1016/j.marchem.2018.05.007, 2018. a
Laakso, L., Mikkonen, S., Drebs, A., Karjalainen, A., Pirinen, P., and Alenius, P.: 100 years of atmospheric and marine observations at the Finnish Utö Island in the Baltic Sea, Ocean Sci., 14, 617–632, https://doi.org/10.5194/os-14-617-2018, 2018. a, b
Lansø, A., Sørensen, L., Christensen, J., Rutgersson, A., and Geels, C.: The influence of short-term variability in surface water pCO2 on modelled air–sea CO2 exchange, Tellus B, 69, 1302670, https://doi.org/10.1080/16000889.2017.1302670, 2017. a, b, c, d
Laws, E. A.: Photosynthetic quotients, new production and net community production in the open ocean, Deep-Sea Res. Pt. A., 38, 143–167, https://doi.org/10.1016/0198-0149(91)90059-O, 1991. a
Lehto, A.-M.: Modelling inorganic carbon system in Utö Baltic Sea, MSc Thesis, University of Helsinki, Helsinki, Finland, 2019. a, b
Liblik, T., and Lips, U.: Stratification has strengthened in the Baltic Sea – An analysis of 35 years of observational data, Front. Earth Sci., 7, 174, https://doi.org/10.3389/feart.2019.00174, 2019. a
Lopez-Urrutia, A., San Martin, E., Harris, R., and Irigoien, X.: Scaling the metabolic balance of the oceans, P. Natl. Acad. Sci. USA, 103, 8739–8744, https://doi.org/10.1073/pnas.0601137103, 2006. a
Millero, F. J.: Carbonate constants for estuarine waters, Mar. Freshwater Res., 61, 139–142, https://doi.org/10.1071/MF09254, 2010. a
Müller, J. D., Schneider, B., and Rehder, G.: Long-term alkalinity trends in the Baltic Sea and their implications for CO2-induced acidification, Limnol. Oceanogr., 61, 1984–2002, https://doi.org/10.1002/lno.10349, 2016. a, b, c, d
Olsen, A., Omar, A. M., Stuart-Menteth, A. C., and Triñanes, J. A.: Diurnal variations of surface ocean pCO2 and sea-air CO2 flux evaluated using remotely sensed data, Geophys. Res. Lett., 31, L20304, https://doi.org/10.1029/2004GL020583, 2004. a
Orr, J. C., Epitalon, J.-M., and Gattuso, J.-P.: Comparison of ten packages that compute ocean carbonate chemistry, Biogeosciences, 12, 1483–1510, https://doi.org/10.5194/bg-12-1483-2015, 2015. a
Redfield, A. C., Ketchum, B. H., and Richards, F. A.: The influence of organisms on the composition of sea-water, Sea, 2, 26–77, 1963. a, b, c
Robinson, C. J.: Microbial respiration, the engine of ocean deoxygenation, Front. Mar. Sci., 5, 533, https://doi.org/10.3389/fmars.2018.00533, 2019. a
Saderne, V., Fietzek, P., and Herman, P. M. J.: Extreme variations of pCO2 and pH in a macrophyte meadow of the Baltic Sea in summer: evidence of the effect of photosynthesis and local upwelling, PLoS ONE, 8, e62689, https://doi.org/10.1371/journal.pone.0062689, 2013. a
Sarmiento, J. L. and Gruber, N.: Ocean Biogeochemical Dynamics, Princeton University Press, New Jersey, USA, 2004. a, b
Schneider, B. and Müller, J. D.: Biogeochemical Transformations in the Baltic Sea: Observations Through Carbon Dioxide Glasses, Springer, Cham, Switzerland, https://doi.org/10.1007/978-3-319-61699-5, 2018. a, b, c, d, e
Schneider, B., Gülzow, W., Sadkowiak, B., and Rehder, G.: Detecting sinks and sources of CO2 and CH4 by ferrybox-based measurements in the Baltic Sea: three case studies, J. Mar. Syst., 140, 13–25, https://doi.org/10.1016/j.jmarsys.2014.03.014, 2014. a
Takahashi, T., Olafsson, J., Goddard, J. G., Chipman, D. W., and Sutherland, S. C.: Seasonal variation of CO2 and nutrients in the high-latitude surface oceans: A comparative study, Global Biogeochem. Cy., 7, 843–878, https://doi.org/10.1029/93GB02263, 1993. a, b, c
Trombetta, T., Vidussi, F., Mas, S., Parin, D., Simier, M., and Mostajir, B.: Water temperature drives phytoplankton blooms in coastal waters, PLoS ONE, 14, e0214933, https://doi.org/10.1371/journal.pone.0214933, 2019. a
Tyrrell, T., Schneider, B., Charalampopoulou, A., and Riebesell, U.: Coccolithophores and calcite saturation state in the Baltic and Black Seas, Biogeosciences, 5, 485–494, https://doi.org/10.5194/bg-5-485-2008, 2008. a, b
van Heuven, S., Pierrot, D., Rae, J., Lewis, E., and Wallace, D. W. R.: CO2SYS v 1.1, MATLAB program developed for CO2 system calculations, ORNL/CDIAC-105b, Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US DoE, Oak Ridge, TN, 2011. a, b
Wanninkhof, R.: Relationship between wind speed and gas exchange over the ocean, J. Geophys. Res., 97, 7373–7382, https://doi.org/10.1029/92JC00188, 1992. a, b
Wanninkhof, R.: Relationship between wind speed and gas exchange over the ocean revisited, Limnol. Oceanogr.-Meth., 12, 351–362, https://doi.org/10.4319/lom.2014.12.351, 2014. a, b, c, d, e, f, g, h, i
Wasmund, N., Andrushaitis, A., Lysiak-Pastuszak, E., Muller-Karulis, B., Nausch, G., Neumann, T., Ojaveer, H., Olenina, I., Postel, L., and Witek, Z.: Trophic status of the south-eastern Baltic Sea: a Comparison of coastal and open areas, Estuar. Coast. Shelf S., 53, 849–864, https://doi.org/10.1006/ecss.2001.0828, 2001. a
Weiss, R. F.: Carbon dioxide in water and seawater: the solubility of a non-ideal gas, Mar. Chem., 2, 203–215, https://doi.org/10.1016/0304-4203(74)90015-2, 1974. a
Wesslander, K., Omstedt, A., and Schneider, B.: Inter-annual and seasonal variations in the air–sea CO2 balance in the central Baltic Sea and the Kattegat, Cont. Shelf Res., 30, 1511–1521, https://doi.org/10.1016/j.csr.2010.05.014, 2010. a
Wesslander, K., Hall, P., Hjalmarsson, S., Lefevre, D., Omstedt, A., Rutgersson, A., Sahlée, E., and Tengberg, A.: Observed carbon dioxide and oxygen dynamics in a Baltic Sea coastal region, J. Mar. Syst., 86, 1–9, https://doi.org/10.1016/j.jmarsys.2011.01.001, 2011. a, b, c, d
Yan, H. Yu, K, Shi, Q., Tan, Y., Liu, G., Zhao, M., Li, S., Chen, T., and Wang, Y.: Seasonal variations of seawater pCO2 and sea-air CO2 fluxes in a fringing coral reef, northern South China Sea, J. Geophys. Res.-Oceans, 121, 998–1008, https://doi.org/10.1002/2015JC011484, 2016. a
Ylöstalo, P., Seppälä, J., Kaitala, S., Maunula, P., and Simis, S.: Loadings of dissolved organic matter and nutrients from the Neva River into the Gulf of Finland – Biogeochemical composition and spatial distribution within the salinity gradient, Mar. Chem., 186, 58–71, https://doi.org/10.1016/j.marchem.2016.07.004, 2016. a
- Abstract
- Introduction
- Materials and methods
- Results and discussion
- Conclusions
- Appendix A: The air–sea exchange of CO2
- Appendix B: The inorganic carbon system
- Appendix C: Total alkalinity
- Data availability
- Author contributions
- Competing interests
- Disclaimer
- Special issue statement
- Acknowledgements
- Financial support
- Review statement
- References
- Abstract
- Introduction
- Materials and methods
- Results and discussion
- Conclusions
- Appendix A: The air–sea exchange of CO2
- Appendix B: The inorganic carbon system
- Appendix C: Total alkalinity
- Data availability
- Author contributions
- Competing interests
- Disclaimer
- Special issue statement
- Acknowledgements
- Financial support
- Review statement
- References