the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Combining benzalkonium chloride addition with filtration to inhibit dissolved inorganic carbon alteration during the preservation of water sample in radiocarbon analysis
Hiroshi A. Takahashi
Masayo Minami
Benzalkonium chloride (BAC) addition has shown great promise as a disinfectant for measuring δ13C and 14C of dissolved inorganic carbon (DIC) in freshwater samples. However, it was reported that the effectiveness of BAC to prevent DIC change was reduced for the use of seawater samples. The present study aims to evaluate the effectiveness of adding BAC as a disinfectant in carbon isotopic analyses of DIC in water samples. We compared the efficacy of BAC addition, filtration (0.22 µm polytetrafluoroethylene (PTFE) or 0.2–0.45 µm polyether sulfone (PES) filters), and a combination of BAC addition and filtration in preventing DIC alterations caused by biological activity using the freshwater (salinity <0.05) and the brackish water (salinity ∼20) samples. The freshwater sample treated with BAC showed no alteration of DIC. In contrast, for the seawater sample, BAC addition alone did not prevent changes in DIC, but the combined treatment was effective. The 14C concentration of samples treated with both BAC addition and filtration exhibited minimal changes, ranging from 0.2–0.4 percent modern carbon (pMC) over 41 weeks despite the addition of sugar included to increase DIC changes several-fold. Although the complete elimination of biological effects may be challenging with the combined method, the observed changes remained within practical limits. Concerns about CO2 contamination during sample filtration were also addressed and found to be negligible. These results suggest that combining filtration and BAC addition is an effective method for suppressing biological DIC alterations in 14C analysis, even in seawater samples.
- Article
(1586 KB) - Full-text XML
-
Supplement
(881 KB) - BibTeX
- EndNote
Radiocarbon (14C) analysis of dissolved inorganic carbon (DIC) in seawater plays a vital role in the elucidation of seawater circulation and atmosphere–ocean CO2 exchange (Matsumoto, 2007; McNichol et al., 2022). For global understanding of ocean water behaviors, it is necessary to analyze samples from various regions over long time frames; ensuring high-quality and consistent analysis is crucial for maintaining data integrity and comparability across different oceans (Key et al., 2002; Anderson, 2020; Olsen et al., 2020). Standard operating procedures (SOPs) for the analysis of seawater have been developed to define the protocols and analytical methods necessary to meet these requirements (Dickson et al., 2007; Abrams, 2013). The SOPs recommend that water samples collected for CO2-related analyses such as DIC, total alkalinity, and CO2 fugacity be treated with a mercuric chloride (HgCl2) solution to prevent biological activity that may alter the carbon distribution in the sample container before analysis. However, the ecological toxicity of HgCl2 poses significant challenges. Additionally, the use of HgCl2 in water samples can lead to uncertainties in the analytical results as mercury interacts with dissolved organic matter in the water, forming complexes that reduce the total alkalinity and potentially complicating the analysis (Mos et al., 2021). Argentino et al. (2023) reported alterations in DIC concentration and δ13C values in marine pore water samples from methane seepage areas treated with HgCl2. Given these environmental and practical concerns, alternative preservation methods that avoid the use of mercury are increasingly desirable.
The methods proposed for the preservation of water samples without the use of HgCl2 include refrigeration, filtration, and the addition of non-toxic or less toxic preservatives (Aucour et al., 1999; Doctor et al., 2008; Ascough et al., 2010; Takahashi et al., 2019a; Wilson et al., 2020; Mos et al., 2021; Takahashi and Minami, 2022). Chemical sterilization methods have been explored, such as adding acids or alkalis to prevent microbial activity in samples intended for the analysis of gases other than CO2, such as methane (Magen et al., 2014). However, altering the pH of water samples is not suitable for DIC analysis as DIC concentrations are highly sensitive to pH changes.
The addition of benzalkonium chloride (BAC) has shown great promise as a disinfectant for measuring δ13C and 14C of DIC in freshwater samples (Takahashi and Minami, 2022; Takahashi et al., 2019a) and for dissolved CH4 concentrations in swamp water (Osaka et al., 2024). BAC is one of the Quaternary ammonium compounds (QACs), a major product of cationic surfactants, and is widely used as a disinfectant (Kuo, 1998; McDonnell and Russell, 1999). QACs penetrate cell membranes and disrupt both the physical and biochemical properties of cells (Gilbert and Moore, 2005; Wessels and Ingmer, 2013). As most of the bioavailable fraction of QAC in environmental waters can be reduced by sewage treatment plants (DeLeo et al., 2020), the ecotoxicological hazard posed by QACs is far lower than that of mercury. Takahashi et al. (2019a) investigated alterations in DIC concentrations and δ13C values in several natural waters (seawater, groundwater, river, pond, and brackish waters) exposed to BAC and beet sugar for about 60 d. They observed that DIC concentrations and δ13C values in freshwater samples remained unaltered throughout the preservation period. In contrast, salty water samples exhibited DIC changes exceeding the analytical error beyond 15 d. Takahashi and Minami (2022) performed a similar assessment of 14C and DIC concentrations in seawater and groundwater. They observed constant 14C and DIC concentrations after 30 d of preservation in groundwater samples, while seawater samples experienced increases in both 14C and DIC concentrations over time. These studies suggest a common trend: seawater samples treated with BAC remain unaltered for a few days but begin to show changes after 1 or 2 weeks. Gloël et al. (2015) examined the impact of BAC addition on the ratio in dissolved gases in seawater. They reported that seawater samples treated with BAC initially showed values identical to those preserved with HgCl2 for the first 3 or 4 d, but changes emerged after 8–17 d. García et al. (2001) found that 50 % of the primary biodegradation of BAC, including benzyl dimethyl tetradecyl ammonium chloride (BAC-C14) and benzyl dimethyl hexadecyl ammonium chloride (BAC-C16), was completed by marine bacterial populations in 8 to >15 d, respectively. Although they did not identify the specific microorganisms responsible for this degradation, their findings align with the observation that BAC's effectiveness in seawater does not persist long term.
Gloël et al. (2015) noted that the factor that diminishes the effectiveness of BAC in seawater over time is likely spores, which are resistant to heat and sterilization. They are highly durable cells that form and lie dormant when bacterial growth conditions deteriorate. Then, as conditions improve because the effectiveness of BAC diminishes, possibly due to interaction with components in the seawater, they can resume growth. The process responsible for reducing BAC's efficacy is unclear, but previous studies mentioned above have suggested that it likely occurs 1–2 weeks after BAC addition to seawater samples. A key factor may be the presence of bacterial spores, but as spores exist universally in both seawater and freshwater (Brown, 2000), their presence alone cannot fully explain the reduced effectiveness of BAC in seawater compared to freshwater. However, a more practical approach would be to focus on removing the spores present in the water sample. Wilson et al. (2020) demonstrated that filtering water samples to 0.2 µm effectively preserves water for 66 d for δ13C measurement of DIC. Spores are primarily produced by aerobic Bacillus species and anaerobic Clostridium species (Brown, 2000), and these rod-shaped bacteria range from approximately 0.2–1 µm in diameter and 1–10 µm in length. As spores are relatively large, they can be easily removed by filtration.
This study aims to determine an effective method for preventing DIC changes caused by biological activity in seawater samples using BAC addition. We evaluate the effectiveness of BAC treatment alone, filtration alone, and the combined use of BAC and filtration for measuring δ13C and 14C of DIC in both freshwater and seawater samples.
2.1 Background of filtration
Filtration can introduce microbubbles, potentially leading to gas dissemination or atmospheric gas exchange. The potential for DIC change during filtration was investigated by comparing the 14C concentrations of NaHCO3 solutions (1 mmol L−1) before and after filtrations. The NaHCO3 solution used here was prepared by diluting a 1 mol L−1 NaHCO3 reagent solution (Kanto Chemical Co. Inc., Japan), which has a low 14C concentration of ∼0.7 percent modern carbon (pMC, Stuiver and Polach, 1977) and a high δ13C value of −3.8 ‰ (Takahashi et al., 2021), with ultrapure water (Milli-Q Direct 8 or Milli-Q Integral 3, Merck Millipore Co., USA). The assessments were carried out using a polyether sulfone (PES) disk filter (25 mm diameter, 0.22 µm pore size; Membrane Solutions Co., Ltd., USA) and a glass fiber (GF) disk filter (25 mm diameter, 1.0 µm pore size; Membrane Solutions Co., Ltd., USA) attached to the syringe (50 mL, disposable syringe; Terumo Corporation, Japan). These samples were designated as NaHCO3-unfiltered, NaHCO3-PES, and NaHCO3-GF, respectively. A common technique employed to impede gas exchange is to introduce the filtered sample water into a sample bottle through a tube, thereby enabling it to overflow. However, in this study, in order to evaluate the maximum impact of filtration, a more drastic technique was employed whereby the filtrate discharged from the filter was directly poured into a beaker without passing through a tube under atmospheric conditions, thereby exposing the sample to atmospheric CO2. The filtration process was conducted on three occasions, once through a PES filter and twice through a GF filter, using the same NaHCO3 solution, and the resulting three filtrates were obtained for each filtration treatment. Immediately after the respective filtrations, the filtrates were injected into reaction containers to extract CO2 from water sample. To ascertain whether any DIC was altered during the filtration experiment, one unfiltered NaHCO3 solution each was introduced into the reaction containers before and after the three filtration treatments (Fig. S1 in the Supplement).
2.2 Filtration, BAC addition, and combined treatment
To compare the efficacy of the various treatments, the following six conditions were evaluated using the natural water samples (SW and GW) mentioned below: (1) no filtration, no BAC addition (control), (2) BAC addition alone (BAC), (3) filtration through a polytetrafluoroethylene (PTFE) filter (PTFE), (4) filtration through a PES filter (PES), (5) PTFE filtration with BAC addition (PTFE+BAC), and (6) PES filtration with BAC addition (PES+BAC). For each assessment of six treatments, the changes in 14C and δ13C during the preservation were quantified on a single occasion. The SW was collected from the sea surface of the Pacific coast at the Nagoya Port, located in Nagoya, Aichi Prefecture, Japan. This site is located near the estuaries of three rivers, the Nikko, Shinkawa, and Shonai rivers, in a tidal flat region. The GW was obtained from a well 80 m deep in Tsukuba, Ibaraki Prefecture, Japan. The chemical composition of the SW (Table S1) indicated that the SW was diluted by river water. The salinities of the SW and GW were determined by summing the chemical composition data to 20.4 and <0.05, respectively (Table S1). While SW can be considered a brackish water sample, it is treated as a coastal seawater sample in this study. For the SW, the expected value of DIC concentration is slightly smaller than 2 mmol L−1, and that of 14C concentration is ∼100 pMC. The DIC and 14C concentrations of groundwater taken from the same well were reported to be 1.69 mmol L−1 and 21.3 ± 0.1 pMC, respectively (Takahashi et al., 2019b; Takahashi and Minami, 2022). At the time of sampling, neither disinfectant treatment nor filtration was applied to any of the natural water samples.
To promote microbial activity and detect even minor changes in DIC that might result from biological processes, beet sugar powder was added in the sample water at a concentration of 2 g L−1 before the preservation of the sample. Given the high 14C concentration of 103.3 ± 0.7 pMC in beet sugar (Takahashi and Minami, 2022), which is approximately equivalent to that of SW, it is conceivable that any 14C changes resulting from the microbial decomposition of beet sugar to DIC might be undetectable in SW samples. To address this, an NaHCO3 solution (1 mol L−1 solution; Kanto Chemical Co. Inc., Japan), which has a low 14C concentration of ∼0.7 pMC and a high δ13C value of −3.8 ‰ (Takahashi et al., 2021), was added to the samples. The addition of NaHCO3 also helped to clarify the δ13C changes in DIC due to beet sugar decomposition as beet sugar exhibits a low δ13C value of −26.2 ‰ (Takahashi and Minami, 2022). Unless otherwise stated, beet sugar and the NaHCO3 solution were added to all water samples.
The treatments were conducted in the following sequence: the NaHCO3 reagent solution (1 mol L−1) was added to the sample waters (∼4 L) at a rate of 2.5 mL L−1 of SW and 2 mL L−1 of GW, effectively doubling the DIC concentration in both water types. This is the (1) control sample. For assessment, (2) BAC, BAC (10 % solution; FUJIFILM Wako Pure Chemical Co., Japan) was added to an aliquot of the sample at a concentration of 0.01 %. Then, the sample waters not used in assessment (2) were filtered using a PTFE or PES disk filter (25 mm diameter, 0.22 µm pore size; Membrane Solutions Co., Ltd.) attached to a syringe (50 mL, disposable syringe) into a beaker. The first 20–30 mL of filtrate was not used for the assessment to pre-wash the filters. These samples were directly utilized for assessments (3) PTFE and (4) PES. For assessments (5) PTFE+BAC and (6) PES+BAC, BAC was added to the retained filtrate at a concentration of 0.01 %. All the treated samples were homogenized in a beaker using a magnetic stirrer. They were then injected into one reaction container for CO2 extraction to obtain the initial 14C concentration and δ13C value and also injected into three preservation bottles (125 mL glass vials) sealed with butyl rubber septa coated with Teflon and aluminum caps, which had been filled with beet sugar powder and evacuated. The water injection into the reaction container and preservation bottles was carried out through a needle attached to a syringe immediately after sample treatments. The preservation periods were 14, 28, and 285 d for SW and 14, 28, and 126 d for GW. At the end of each preservation period, the bottles were opened one by one, and the CO2 was extracted (Fig. S2). Before experiments, the vials and butyl rubber septa were sterilized by heating at 450 °C for 6 h and by autoclaving, respectively. The BAC used in this study primarily consisted of benzyl dimethyl dodecyl ammonium chloride (BAC-C12) and benzyl dimethyl tetradecyl ammonium chloride (BAC-C14).
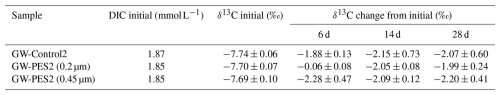
Since the filters for treatments (3)–(6) were not sterilized, additional assessments were conducted using sterilized PES disk filters (25 mm diameter, 0.2 µm and 0.45 µm pore sizes, GVS Japan). GW samples, with or without filtration, were preserved in 34 mL glass vials for 6, 14, and 28 d. These treatments were labeled GW-Control2, GW-PES2 (0.2 µm), and GW-PES2 (0.45 µm), respectively (Fig. S3). A total of 12 bottles were prepared for each treatment, with three bottles used for the initial value and each of the three preservation periods to measure δ13C values by GC-IRMS. As these samples were not analyzed for 14C, the NaHCO3 solution to lower the initial 14C concentration was not added. Other procedures were the same as for treatments (3) and (4).
2.3 14C concentration and δ13C measurements
CO2 extraction from water samples for the measurement of 14C concentration and δ13C values was performed using the ReCEIT (repeated cycles of extraction, introduction, and trapping) method (Takahashi et al., 2021), which is a simple and carrier-gas-free method that can handle a variety of water samples with a wide range of DIC concentrations (0.4–100 mmol L−1 in the case of 1 mmol of carbon) and produces high CO2 yields. The procedure is composed of repeating the cycles of CO2 extraction from water into the headspace of the reaction container, expansion of the extracted gas into the vacuum line, and cryogenic trapping of CO2. This method, which extracts CO2 without bubbling, is particularly well suited for BAC-added samples, which tend to foam. An approximate DIC concentration was calculated from the volume of water treated and the CO2 extracted. The CO2 gas was reduced to graphite (Kitagawa et al., 1993) for analysis by accelerator mass spectrometry (AMS) following the removal of sulfur oxide gas using the Sulfix reagent (8–20 mesh; Kishida Chemical Co., Ltd., Japan) as necessary. The 14C concentrations were measured using a 3 MV AMS (Model 4130-AMS, HVEE, the Netherlands) at the Institute for Space–Earth Environmental Research, Nagoya University, Japan (Nakamura et al., 2000), and a 1 MV AMS (4110Bo-AMS-3, HVEE, the Netherlands) at the Korea Institute of Geoscience and Mineral Resources (KIGAM), South Korea (Hong et al., 2010). Corrections for isotopic fractionation (Stuiver and Polach, 1977) were performed using the ratio measured by AMS. The standard deviations for 14C measurements were 0.02–0.04 pMC for waters with concentrations below 1 pMC and less than 0.8 % of 14C concentration for waters above 10 pMC when measured at Nagoya University. The precision of the quantitative analysis of carbon was better than 3 %, and the background was below at KIGAM. The error in 14C measurement was represented in 1σ based on a standard calculation method in 14C analysis (Scott et al., 2007).
The δ13C values of CO2 gas extracted by the ReCEIT procedure were determined via isotope-ratio mass spectrometry (IRMS) with a dual inlet system (Delta-V Advantage, Thermo Fisher Scientific, Inc., USA) at the Geological Survey of Japan. The standard deviation of multiple δ13C measurements by IRMS is less than 0.01 ‰, and for individual measurements, the error is represented as 1σ calculated from the variations in the dual inlet measurement. Some δ13C measurements were performed using a continuous-flow IRMS coupled to a gas chromatography system (GC-IRMS; Delta-V Advantage with Gas Bench II, Thermo Fisher Scientific, Inc., USA) at the Geological Survey of Japan (Takahashi et al., 2019a). The standard deviation of multiple measurements of water samples by GC-IRMS is 0.04 ‰ (1σ). CO2 gas was extracted from water by addition of phosphoric acid in a septum-sealed Exetainers® vial (12 mL; Labco Ltd., UK). The δ13C value of each sample is represented as the averaged value over the three vials with the standard deviation (1σ).
3.1 Background on the 14C concentration and δ13C values in the filtration treatment process
The 14C concentrations and δ13C values of two NaHCO3-unfiltered samples, both before and after filtration assessments, were identical, indicating that the DIC of the NaHCO3 solution itself remained unchanged during the assessment experiment (Fig. 1, Table S2). The 14C concentrations of NaHCO3-unfiltered, NaHCO3-PES, and NaHCO3-GF were consistent within the error range. Although each value was obtained from a single experiment, the five analytical results showed high consistency, indicating no detectable change in 14C concentration due to filtration. This suggests that any increase in 14C due to CO2 contamination during filtration was not a significant concern. In contrast, the δ13C values showed a very slight decrease for NaHCO3-PES and NaHCO3-GF (Fig. 1, Table S2). This slight change in δ13C is assumed to be caused by atmospheric CO2 contamination or CO2 degassing from the NaHCO3 solution during filtration. If atmospheric CO2 contamination caused the δ13C shift in the NaHCO3 solution, the 14C concentration would vary according to the amount of atmospheric CO2 contamination. Assuming atmospheric CO2 has a δ13C value of −10 ‰ and a 14C concentration of 100 pMC, the 14C concentrations of NaHCO3-PES and two NaHCO3-GF could be calculated as 3.6 ± 0.2 pMC, 4.5 ± 0.2 pMC, and 2.4 ± 0.2 pMC, respectively. These calculated values do not align with the measured 14C concentrations, suggesting that atmospheric CO2 contamination is unlikely.
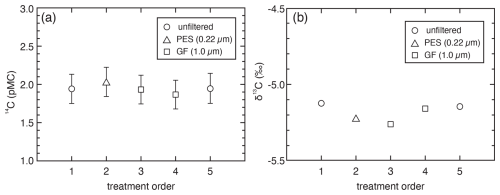
Figure 1Comparisons of 14C (a) and δ13C (b) among the unfiltered and filtered solutions of 1 mmol L−1 of NaHCO3. PES: filtered by a PES disk filter (25 mm in diameter, 0.22 µm in pore size). GF: filtered by GF disk filter (25 mm in diameter, 1.0 µm in pore size). Each value of 14C and δ13C represents a single treatment occasion. The bars of 14C concentration represent the measurement error of the AMS analysis. The δ13C error is smaller than the size of the plotting symbols.
The δ13C of DIC would change due to isotope fractionation associated with degassing. When DIC and gaseous CO2 are in isotopic equilibrium, the δ13C of DIC is typically higher than that of gaseous CO2 (Zhang et al., 1995). As carbon with a lower δ13C value is removed as CO2 during degassing, the δ13C of the remaining DIC in the solution would gradually increase. However, the measured δ13C showed the opposite trend, indicating that the change in δ13C is not due to CO2 degassing from the NaHCO3 solution. Thus, the two hypotheses – that δ13C changes were caused by atmospheric CO2 contamination or by degassing – were rejected, and the observed δ13C change may be attributed to an unidentified artifact factor other than filtration. Carbon contamination during sample treatments could significantly influence 14C analysis, but the impact of isotopic fractionation can be eliminated by corrective calculations. Since the primary objective of this study is 14C analysis, the effect of filtration is expected to be minimal or negligible. However, if δ13C analyses were conducted, careful scrutiny and verification would be necessary. The experimental procedure in this study was designed to evaluate the maximum impact of filtration, and it is anticipated that the impact can be minimized by adopting experimental procedures that minimize filtration-related effects.
As the filtration in this assessment was performed under atmospheric conditions with CO2 exposure, it was likely to cause carbon contamination. However, the identical 14C concentrations (Fig. 1) suggest that a 14C increase due to CO2 contamination during filtration should not be considered a concern. Nonetheless, depending on the filter material or pore size, the water sample may not pass through unless the syringe is pressed forcefully, which can lead to contamination by the atmospheric CO2 inside the syringe. When filtration was performed with a 1 mmol L−1 NaHCO3 solution and an equal volume of air inside the syringe using a PES filter (0.22 µm), the 14C concentration of the NaHCO3 solution was measured to increase by 0.7 pMC, rising to 4.6 pMC from an initial 3.9 pMC in our assessment. This 14C increase is quantitatively reasonable, assuming a CO2 concentration of 400 ppm and that the CO2 inside the syringe fully dissolved into the NaHCO3 solution. It is important to remove air bubbles in the syringe at the filtration.
3.2 14C concentration and δ13C changes in natural water samples
The initial values of DIC concentrations, 14C concentrations, and δ13C values for SW mixed with the NaHCO3 solution were 3.60–3.65 mmol L−1, 41.2–42.2 pMC, and −4.05 ‰ to −3.72 ‰, respectively (Table S3). For GW, these values were 4.48–4.93 mmol L−1, 10.2–10.9 pMC, and −6.00 ‰ to −5.98 ‰ when mixed with the NaHCO3 solution and 1.85–1.87 mmol L−1 and −7.74 ‰ to −7.69 ‰ when not mixed with the NaHCO3 solution (Table S1), respectively. After mixing with NaHCO3, the 14C concentrations in both SW and GW were approximately half or slightly less than half of their original concentrations.
The largest changes in 14C and δ13C during the preservation period were observed in the control samples, with progressively smaller changes occurring in the order of filtration-only samples, BAC-only samples, and those treated with both filtration and BAC. The 14C concentrations increased as the preservation period lengthened for SW-Control, GW-Control, SW-PES, GW-PES, SW-PTFE, GW-PTFE, and SW-BAC (Fig. 2). It is reasonable to assume that these large changes in 14C concentration and δ13C were caused by the DIC derived from beet sugar given that beet sugar is more easily degraded than BAC or other organic materials suspended in water. Given that DIC change during the preservation was enhanced by the incorporation of sugar, it is imperative to ascertain the impact of sugar addition. Takahashi and Minami (2022) defined the boost effect as an index of how many times the DIC change in the sugar-added sample is greater than the DIC concentration change in the no-sugar sample during the preservation. It is anticipated that the boost effect will be more pronounced in instances where there is a paucity of organic matter and a greater prevalence of microorganisms in the water sample. The SW in this study was sampled at a tidal flat location along the Pacific coast, near the estuaries of major rivers. It can be reasonably assumed that water discharged from tidal flats will have higher concentrations of organic carbon, nutrients, and microbes than typical seawater (Sakamaki et al., 2006; Hu et al., 2016). Accordingly, the boost effect of SW in this study may be identical to or slightly smaller than 3.0 ± 1.4, as reported by Takahashi and Minami (2022) for the seawater sample sampled at Kashima Port on the Pacific coast. This seawater was not mixed with river waters and was not sampled from the tidal flat. The boost effect of groundwater sampled from the same well as the GW was reported to be 5.3 ± 1.8. As the exact boost effect is not measured in the present study, the increase in DIC due to sugar addition was not corrected through calculation. It is important to note that the described DIC change includes an increase of probably 2 or 3 times for SW and 5 times for GW caused by the addition of sugar.
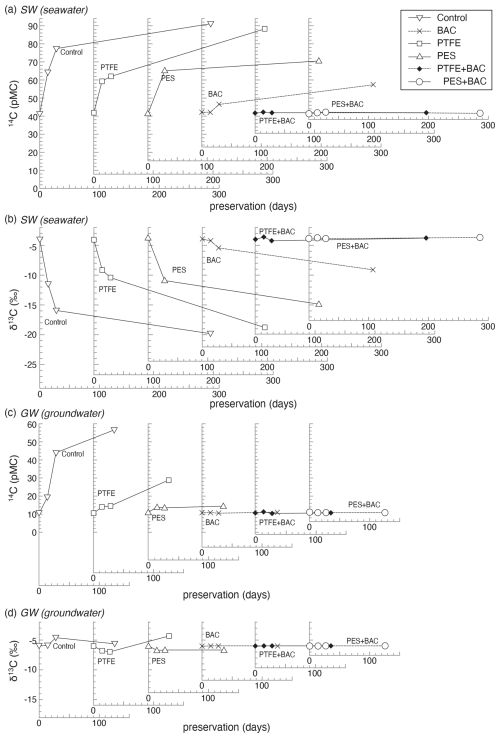
Figure 2Changes in 14C and δ13C during the preservation of SW and GW mixed with a NaHCO3 solution and beet sugar. Changes in DIC were augmented by the addition of beet sugar. (a) 14C of SW, (b) δ13C of SW, (c) 14C of GW, and (d) δ13C of GW. The time series of DIC change for the respective treatments were derived from each experiment conducted on a single sample with varying preservation periods. The errors in 14C and δ13C are equivalent to or smaller than the size of the plotting symbols.
3.3 Comparison of treatments
Filtration, BAC addition, and the combined treatment indicated more effective than the control samples in preserving DIC in water samples (Fig. 2). However, in some cases, changes could not be completely reduced, depending on the type of treatment or the water used. In samples treated with BAC alone, the results were consistent with previous studies (Takahashi et al., 2019a; Takahashi and Minami, 2022), where GW showed suppression of DIC changes, but SW showed changes of 15.1 pMC in 14C concentration and −5.2 ‰ in δ13C. This confirms that BAC alone is not suitable for seawater samples and indicates that the seawater sample utilized in this investigation contains constituents, probably unique microorganisms, whose biological activity cannot be entirely suppressed by BAC addition, as reported in previous studies. Accordingly, SW represents an appropriate sample for the purpose of investigating potential methods for addressing the issue of BAC impairment in seawater. The 14C concentrations and δ13C values were observed to be relatively constant in samples treated with both filtration and BAC: SW-PTFE+BAC, SW-PES+BAC, GW-BAC, GW-PTFE+BAC, and GW-PES+BAC. The 14C and δ13C changes were minimal for SW-PTFE+BAC, SW-PES+BAC, GW-BAC, GW-PTFE+BAC, and GW-PES+BAC (Table S3). Since only a single time series of data is available for each treatment, the actual values for DIC changes remain uncertain. However, as the comparison is based on time series data, it can be posited that treatments exhibiting minimal change are highly effective.
The 14C concentration and δ13C value of filtered waters without BAC addition showed significant changes although they were smaller than those in unfiltered samples. The 14C changes in SW-Control, SW-PTFE, and SW-PES were 50.0, 46.4, and 29.2 pMC, respectively, while those of GW-Control, GW-PTFE, and GW-PES were 46.1, 18.3, and 3.6 pMC, respectively. DIC changes were smaller with PES filtration than PTFE for both SW and GW despite using the same pore-size filter. This may be related to the fact that PTFE has higher resistance than PES, requiring more force during filtration.
The changes in δ13C of the GW-PES2 as the sample filtered through a sterile filter were consistent with those of GW-Control2 as the unfiltered sample, except for a slight change in GW-PES2 (0.2 µm) after 6 d (Table 1). This suggests that DIC changes in the filtered samples shown in Fig. 2 were not caused by the microorganisms derived from the filter. While using a 0.2 µm filter reduces the number of microorganisms compared with a 0.45 µm filter, once some slip through, the difference between filters may disappear as the microorganisms proliferate. Wilson et al. (2020) reported that filtration alone is sufficient to prevent DIC changes due to its biological activity. However, our results showed that this method was insufficient as DIC changes could not be ignored for SW-PTFE, SW-PES, and GW-PES2 (0.2 µm) with preservation periods longer than 14 d, although this study confirmed that filtration reduces DIC changes in water samples during preservation (Fig. 2, Table 1). This was especially the case for GW-PES2 (0.45 µm) samples, where sugar addition increased biological activity. Sugar addition may have artificially triggered microbial growth, resulting in DIC changes that would not have occurred otherwise. Without microbial growth triggers, filtration may be more effective, but DIC changes were smaller with SW-BAC compared to SW-PTFE or SW-PES. Therefore, BAC addition is more effective than filtration alone in reducing DIC changes although it has the disadvantage of not being able to use the sample for other analyses.
3.4 Combined treatment of BAC addition and filtration
The combined treatments, PTFE+BAC and PES+BAC, showed consistent 14C concentrations within the analytical error during preservation for both SW and GW. Though slight δ13C changes were observed in SW, this δ13C change seems to be negligible given its small magnitude and uncertainty, which only became detectable through sugar-induced microbial activity magnification.
One possible explanation for the minimal DIC changes in the combined treatment may be due to effectiveness of BAC was enhanced by reducing the number of microorganisms through filtration. This explains why no DIC changes were observed in SW-PTFE+BAC and SW-PES+BAC during the preservation period. In contrast, the DIC change observed in SW-BAC may be caused by BAC being insufficient against the number of microorganisms present; however, the reason why the change was observed during the only second half of the preservation period cannot be explained. If microorganisms are killed by BAC, reactivation should not occur in the second half of the preservation period. As mentioned in the Introduction, it has been suggested that the lower effectiveness of BAC in seawater may be due to spores (Gloël et al., 2015) that cannot be effectively inactivated by BAC. They are not significantly different in size from rod-shaped bacteria, the main spore-forming microorganisms (Brown, 2000), and range in size from 0.6–4 µm (Reponen et al., 2001). These larger microorganisms are likely to be removed by filtration. Our assessment indicated that filtration alone might allow some microorganisms to pass through, but BAC can inactivate the vegetative cells of small microorganisms. Filtration removes larger spores that may cause DIC changes in seawater. The role of spores has not been fully verified, but this could explain why DIC in SW-BAC remained unchanged until 14 d and changed after 28 d.
Certain bacteria can degrade QACs (García et al., 2001; Patrauchan and Oriel, 2003; Zhang et al., 2011; Oh et al., 2013). If the water sample contained microorganisms capable of degrading BAC, biological activity would not be inhibited, leading to DIC changes. While BAC may kill most microorganisms, BAC-tolerant microorganisms could survive and recover, causing detectable DIC changes. This could explain the DIC change observed in SW-BAC. If BAC-tolerant microorganisms were removed by filtration, it would align with the lack of DIC changes in SW-PTFE+BAC and SW-PES+BAC. Microorganisms reported to adapt to BAC and cause biodegradation include Pseudomonas, Aeromonas hydrophila, Salmonella enterica, and Klebsiella oxytoca (Ferreira et al., 2011; Khan et al., 2015; Cui et al., 2023). These species are commonly found in aquatic environments. These microorganisms are not originally tolerant to BAC but gradually adapt over long periods, such as several tens of days of exposure (Oh et al., 2013; Yang et al., 2023). Preservation in sealed vials without aeration for DIC analysis is unlikely to permit the adaptation of microorganisms to BAC due to insufficient exposure time. Thus, for BAC biodegradation to occur, microorganisms must be initially tolerant of BAC in the water sample. QACs in water are removed by microbial communities tolerant to them, often found in sewage treatment plants (Zhang et al., 2015; DeLeo et al., 2020). Previous biodegradation studies have isolated microbial communities from enriched cultures grown on BAC-based media or activated sludge (Chacón et al., 2023). Therefore, it cannot be ruled out that microorganisms tolerant to BAC might be present in estuary and coastal waters where sewage effluents mix. However, degradation of BAC, a refractory organic compound, only begins after readily decomposable organic matter is fully consumed (Zhang et al., 2011). If BAC-tolerant microorganisms were present, beet sugar would be consumed first, and the 14C and δ13C of DIC in preserved water samples would reflect beet sugar more closely than BAC. Even in this case, the lack of DIC changes in the SW-PTFE+BAC and SW-PES+BAC samples indicates that filtration is effective at removing such microorganisms. When combining filtration and BAC addition, avoiding contamination with ambient carbon (atmospheric CO2) during filtration is essential. A blank check with NaHCO3 demonstrated that the 14C background remained unchanged, confirming that filtration represents a viable process provided that the necessary precautions are properly followed. As a consequence of ongoing technological advancements, the quantity of carbon required for 14C measurement by AMS is progressively diminishing (Minami et al., 2013; Ruff et al., 2010). The reduction in sample size facilitates filtration and minimizes background contamination, which represents a favorable development for this combined procedure.
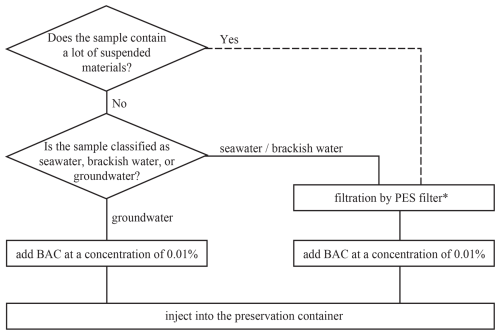
Figure 3Summary flowchart for the preservation of water samples for radiocarbon measurement in DIC. Dotted line: although not directly verified in this study, filtration is recommended based on the reporting of Zhang et al. (2015), which suggests that BAC can be removed by adsorption to particles in water. *: filters with a pore size of 0.22 µm were evaluated in this study; while PTFE filters were effective, they showed a significantly higher resistance to filtration compared to PES filters. As a result, PES filters are expected to induce fewer changes in DIC.
This study assessed several treatments aimed at suppressing changes in DIC during sample preservation as an alternative to HgCl2 addition. We found that the combination of filtration and BAC addition effectively inhibited DIC changes due to microbial activity during preservation. In water samples treated with this method, DIC changes were minimal even when sugar was added to significantly enhance microbial activity. In practical analyses, such a boost from adding sugar would not occur, leading to the conclusion that the combined method of BAC addition followed by filtration is an effective procedure for inhibiting DIC changes caused by biological activity. The slight changes in DIC observed in BAC-supplemented seawater samples may be attributed to microorganisms that BAC could not inactivate. They are presumed to be spores or BAC-tolerant microorganisms. The size of spores and spore-forming microorganisms varies among species, but they are generally large enough to be removed through filtration. Many microorganisms that adapt to BAC are also relatively large and can be removed by filtration.
DIC changes in seawater samples can be suppressed through a two-step process: first, filtration to remove spores or microorganisms tolerant to BAC (if present), which is followed by BAC treatment to inactivate microorganisms that passed through filtration. It should be noted that smaller microorganisms may still pass through the filtration system. In some cases, the partial removal of microorganisms through filtration may not fully suppress the DIC changes, leading to microorganism recovery within a few days, resulting in DIC changes similar to those in the unfiltered samples. Therefore, careful consideration of the preservation period is necessary when using filtration alone to suppress DIC changes.
We recommend a combined treatment of filtration and BAC addition to suppress DIC changes during sample preservation (Fig. 3) as it offers a safer alternative to HgCl2. In this study, a 0.22 µm pore size filter is used to validate earlier findings. However, it is likely that a filter with a coarser pore size could also remove spores given their size. Verifying the optimal pore size is an important next step. As observed in this study, using a very fine pore-size filter can slow sample flow, increase resistance, impair operability, and elevate blanks. Future research should include blank verifications of the combined technique and further verification of whether the slight DIC change observed here can be detected in other water samples. It should also be noted that BAC in water may be removed primarily by adsorption onto sludge rather than by biodegradation (Zhang et al., 2015). Our preliminary result showed that the bactericidal efficiency of BAC was likely diminished in the water sample containing muddy sediment. While filtration can remove sludge or sediment, caution is needed when applying the combined treatment to water samples containing large amounts of suspended material. In such cases, increasing the amount of BAC may be necessary for effective treatment. The assessment of this combined procedure was conducted on a limited number of natural water samples, and further investigation into the optimal filter pore size and verification using other natural water samples are necessary. However, this procedure offers a practical and environmentally friendly alternative to conventional mercury-disinfected methods for water sample preservation in aquatic environments.
The data utilized in this study are presented in Table 1 in the paper and Tables S1–3 in the Supplement.
The supplement related to this article is available online at https://doi.org/10.5194/os-21-1395-2025-supplement.
HAT participated in the design and discussion of the study as well as in the δ13C measurements. MM contributed to the discussion and to the 14C measurements.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
The authors thank Hiroyuki Kitagawa of Nagoya University and Wan Hong of KIGAM for their help with the AMS measurements. We are also grateful to Hiroko Handa of the Geological Survey of Japan, AIST (affiliated at the time of the experiment), and Koh Kakiuchida of Nagoya University for their experimental support of CO2 extraction and Akihiko Inamura of the Geological Survey of Japan, AIST, for his help with the ion chromatography measurements. The authors also sincerely thank the anonymous reviewers for their time and thoughtful feedback on our manuscript. Part of this study was carried out by the joint research program of the Institute for Space–Earth Environmental Research, Nagoya University.
This research has been supported by the Japan Society for the Promotion of Science KAKENHI (grant no. 23K03500).
This paper was edited by Xinping Hu and reviewed by three anonymous referees.
Abrams, M.: Best Practices for the Collection, Analysis, and Interpretation of Seabed Geochemical Samples To Evaluate Subsurface Hydrocarbon Generation and Entrapment, Offshore Technology Conference, Houston, Texas, USA, May 2013, https://doi.org/10.4043/24219-MS, 2013.
Anderson, R. F.: GEOTRACES: Accelerating research on the marine biogeochemical cycles of trace elements and their isotopes, Annu. Rev. Mar. Sci., 12, 49–85, https://doi.org/10.1146/annurev-marine-010318-095123, 2020.
Argentino, C., Kalenitchenko, D., Lindgren, M., and Panieri, G.: HgCl2 addition to pore water samples from cold seeps can affect the geochemistry of dissolved inorganic carbon ([DIC], δ13CDIC), Mar. Chem., 251, 104236, https://doi.org/10.1016/j.marchem.2023.104236, 2023.
Ascough, P. L., Cook, G. T., Church, M. J., Dunbar, E., Einarsson, A., McGovern, T. H., Dugmore, A. J., Perdikaris, S., Hastie, H., Frioriksson, A., and Gestsdottir, H.: Temporal and spatial variations in freshwater 14C reservoir effects: Lake Mývatn, northern Iceland, Radiocarbon, 52, 1098–1112, https://doi.org/10.1017/S003382220004618X, 2010.
Aucour, A. M., Sheppard, S. M. F., Guyomar, O., and Wattelet, J.: Use of 13C to trace origin and cycling of inorganic carbon in the Rhône river system, Chem. Geol., 159, 87–105, https://doi.org/10.1016/S0009-2541(99)00035-2, 1999.
Brown, K. L.: Control of bacterial spores, Brit. Med. Bull., 56, 158–171, https://doi.org/10.1258/0007142001902860, 2000.
Chacón, L., Kuropka, B., González-Tortuero, E., Schreiber, F., Rojas-Jiménez, K., and Rodríguez-Rojas, A.: Mechanisms of low susceptibility to the disinfectant benzalkonium chloride in a multidrug-resistant environmental isolate of, Front. Microbiol., 14, 1180128, https://doi.org/10.3389/fmicb.2023.1180128, 2023.
Cui, Y. C., Gao, J. F., Zhao, M. Y., Guo, Y., Zhao, Y. F., and Wang, Z. Q.: Deciphering the interaction impacts between antiseptic benzethonium chloride and biofilm nitrification system: Performance, resistance mechanisms and biodegradation, Water Res., 240, 120062, https://doi.org/10.1016/j.watres.2023.120062, 2023.
DeLeo, P. C., Huynh, C., Pattanayek, M., Schmid, K. C., and Pechacek, N.: Assessment of ecological hazards and environmental fate of disinfectant quaternary ammonium compounds, Ecotox. Environ. Safe., 206, 111116, https://doi.org/10.1016/j.ecoenv.2020.111116, 2020.
Dickson, A. G., Sabine, C. L., and Christian, J. R. (Eds.): Guide to best practices for ocean CO2 measurements, PICES Special Publication 3; IOCCP Report 8, North Pacific Marine Science Organization, Sidney, British Columbia, 191 pp., https://doi.org/10.25607/OBP-1342, 2007.
Doctor, D. H., Kendall, C., Sebestyen, S. D., Shanley, J. B., Ohte, N., and Boyer, E. W.: Carbon isotope fractionation of dissolved inorganic carbon (DIC) due to outgassing of carbon dioxide from a headwater stream, Hydrol. Process., 22, 2410–2423, https://doi.org/10.1002/hyp.6833, 2008.
Ferreira, C., Pereira, A. M., Pereira, M. C., Melo, L. F., and Simoes, M.: Physiological changes induced by the quaternary ammonium compound benzyldimethyldodecylammonium chloride on Pseudomonas fluorescens, J. Antimicrob. Chemoth., 66, 1036–1043, https://doi.org/10.1093/jac/dkr028, 2011.
García, M. T., Ribosa, I., Guindulain, T., Sánchez-Leal, J., and Vives-Rego, J.: Fate and effect of monoalkyl quaternary ammonium surfactants in the aquatic environment, Environ. Pollut., 111, 169–175, https://doi.org/10.1016/S0269-7491(99)00322-X, 2001.
Gilbert, P. and Moore, L. E.: Cationic antiseptics: diversity of action under a common epithet, J. Appl. Microbiol., 99, 703–715, https://doi.org/10.1111/j.1365-2672.2005.02664.x, 2005.
Gloël, J., Robinson, C., Tilstone, G. H., Tarran, G., and Kaiser, J.: Technical note: Could benzalkonium chloride be a suitable alternative to mercuric chloride for preservation of seawater samples?, Ocean Sci., 11, 947–952, https://doi.org/10.5194/os-11-947-2015, 2015.
Hong, W., Park, J. H., Sung, K. S., Woo, H. J., Kim, J. K., Choi, H. W., and Kim, G. D.: A new 1mv AMS facility at KIGAM, Radiocarbon, 52, 243–251, https://doi.org/10.1017/S0033822200045276, 2010.
Hu, Y., Wang, L., Fu, X. H., Yan, J. F., Wu, J. H., Tsang, Y. F., Le, Y. Q., and Sun, Y.: Salinity and nutrient contents of tidal water affects soil respiration and carbon sequestration of high and low tidal flats of Jiuduansha wetlands in different ways, Sci. Total Environ., 565, 637–648, https://doi.org/10.1016/j.scitotenv.2016.05.004, 2016.
Key, R. M., Quay, P. D., Schlosser, P., McNichol, A. P., von Reden, K. F., Schneider, R. J., Elder, K. L., Stuiver, M., and Ostlund, H. G.: WOCE radiocarbon IV: Pacific Ocean results; P10, P13N, P14C, P18, P19 & S4P, Radiocarbon, 44, 239–392, https://doi.org/10.1017/S0033822200064845, 2002.
Khan, A. H., Topp, E., Scott, A., Sumarah, M., Macfie, S. M., and Ray, M. B.: Biodegradation of benzalkonium chlorides singly and in mixtures by a Pseudomonas sp. isolated from returned activated sludge, J. Hazard. Mater., 299, 595–602, https://doi.org/10.1016/j.jhazmat.2015.07.073, 2015.
Kitagawa, H., Masuzawa, T., Makamura, T., and Matsumoto, E.: A batch preparation method for graphite targets with low-background for AMS 14C measurements, Radiocarbon, 35, 295–300, https://doi.org/10.1017/S0033822200064973, 1993.
Kuo, C. Y.: Improved application of ion chromatographic determination of carboxylic acids in ozonated drinking water, J. Chromatogr. A, 804, 265–272, https://doi.org/10.1016/S0021-9673(98)00089-2, 1998.
Magen, C., Lapham, L. L., Pohlman, J. W., Marshall, K., Bosman, S., Casso, M., and Chanton, J. P.: A simple headspace equilibration method for measuring dissolved methane, Limnol. Oceanogr.-Meth., 12, 637–650, https://doi.org/10.4319/lom.2014.12.637, 2014.
Matsumoto, K.: Radiocarbon-based circulation age of the world oceans, J. Geophys. Res., 112, C09004, https://doi.org/10.1029/2007jc004095, 2007.
McDonnell, G. and Russell, A. D.: Antiseptics and disinfectants: Activity, action, and resistance, Clin. Microbiol. Rev., 12, 147–179, https://doi.org/10.1128/Cmr.12.1.147, 1999.
McNichol, A. P., Key, R. M., and Guilderson, T. P.: Global ocean radiocarbon programs, Radiocarbon, 64, 675–687, https://doi.org/10.1017/Rdc.2022.17, 2022.
Minami, M., Kato, T., Miyata, Y., Nakamura, T., and Hua, Q.: Small-mass AMS radiocarbon analysis at Nagoya University, Nucl. Instrum. Meth. B, 294, 91–96, https://doi.org/10.1016/j.nimb.2012.02.036, 2013.
Mos, B., Holloway, C., Kelaher, B. P., Santos, I. R., and Dworjanyn, S. A.: Alkalinity of diverse water samples can be altered by mercury preservation and borosilicate vial storage, Sci. Rep.-UK, 11, 9961, https://doi.org/10.1038/s41598-021-89110-w, 2021.
Nakamura, T., Niu, E., Oda, H., Ikeda, A., Minami, M., Takahashi, H., Adachi, M., Pals, L., Gottdang, A., and Suya, N.: The HVEE Tandetron AMS system at Nagoya University, Nucl. Instrum. Meth. B, 172, 52–57, https://doi.org/10.1016/S0168-583x(00)00398-0, 2000.
Oh, S., Tandukar, M., Pavlostathis, S. G., Chain, P. S. G., and Konstantinidis, K. T.: Microbial community adaptation to quaternary ammonium biocides as revealed by metagenomics, Environ. Microbiol., 15, 2850–2864, https://doi.org/10.1111/1462-2920.12154, 2013.
Olsen, A., Lange, N., Key, R. M., Tanhua, T., Bittig, H. C., Kozyr, A., Álvarez, M., Azetsu-Scott, K., Becker, S., Brown, P. J., Carter, B. R., Cotrim da Cunha, L., Feely, R. A., van Heuven, S., Hoppema, M., Ishii, M., Jeansson, E., Jutterström, S., Landa, C. S., Lauvset, S. K., Michaelis, P., Murata, A., Pérez, F. F., Pfeil, B., Schirnick, C., Steinfeldt, R., Suzuki, T., Tilbrook, B., Velo, A., Wanninkhof, R., and Woosley, R. J.: An updated version of the global interior ocean biogeochemical data product, GLODAPv2.2020, Earth Syst. Sci. Data, 12, 3653–3678, https://doi.org/10.5194/essd-12-3653-2020, 2020.
Osaka, K., Nagata, R., Inoue, M., Itoh, M., Hosoi-Tanabe, S., and Iwata, H.: A simple, safe method for preserving dissolved methane in freshwater samples using benzalkonium chloride, Limnol. Oceanogr.-Meth., 22, 536–547, https://doi.org/10.1002/lom3.10632, 2024.
Patrauchan, M. A. and Oriel, P. J.: Degradation of benzyldimethylalkylammonium chloride by Aeromonas hydrophila sp. K., J. Appl. Microbiol., 94, 266–272, https://doi.org/10.1046/j.1365-2672.2003.01829.x, 2003.
Reponen, T., Grinshpun, S. A., Conwell, K. L., Wiest, J., and Anderson, W. J.: Aerodynamic versus physical size of spores: Measurement and implication for respiratory deposition, Grana, 40, 119–125, https://doi.org/10.1080/00173130152625851, 2001.
Ruff, M., Fahrni, S., Gäggeler, H. W., Hajdas, I., Suter, M., Synal, H. A., Szidat, S., and Wacker, L.: On-line radiocarbon measurements of small samples using elemental analyzer and MICADAS gas ion source, Radiocarbon, 52, 1645–1656, https://doi.org/10.1017/S003382220005637x, 2010.
Sakamaki, T., Nishimura, O., and Sudo, R.: Tidal time-scale variation in nutrient flux across the sediment-water interface of an estuarine tidal flat, Estuar. Coast. Shelf S., 67, 653–663, https://doi.org/10.1016/j.ecss.2006.01.005, 2006.
Scott, E. M., Cook, G. T., and Naysmith, P.: Error and uncertainnty in radiocarbon measurements, Radiocarbon, 49, 427–440, https://doi.org/10.1017/S0033822200042351, 2007.
Stuiver, M. and Polach, H. A.: Reporting of 14C data – Discussion, Radiocarbon, 19, 355–363, https://doi.org/10.1017/S0033822200003672, 1977.
Takahashi, H. A. and Minami, M.: Assessment of the influence of benzalkonium chloride addition on radiocarbon analysis of dissolved inorganic carbon, Limnol. Oceanogr.-Meth., 20, 605–617, https://doi.org/10.1002/lom3.10508, 2022.
Takahashi, H. A., Handa, H., Sugiyama, A., Matsushita, M., Kondo, M., Kimura, H., and Tusujimura, M.: Filtration and exposure to benzalkonium chloride or sodium chloride to preserve water samples for dissolved inorganic carbon analysis, Geochem. J., 53, 305–318, https://doi.org/10.2343/geochemj.2.0570, 2019a.
Takahashi, H. A., Minami, M., Aramaki, T., Handa, H., and Matsushita, M.: Radiocarbon changes of unpoisoned water samples during long-term storage, Nucl. Instrum. Meth. B, 455, 195–200, https://doi.org/10.1016/j.nimb.2018.11.029, 2019b.
Takahashi, H. A., Handa, H., and Minami, M.: A simple CO2 extraction method for radiocarbon analyses of dissolved inorganic carbon in water samples without a carrier gas, Radiocarbon, 63, 1339–1353, https://doi.org/10.1017/RDC.2021.48, 2021.
Wessels, S. and Ingmer, H.: Modes of action of three disinfectant active substances: A review, Regul. Toxicol. Pharm., 67, 456–467, https://doi.org/10.1016/j.yrtph.2013.09.006, 2013.
Wilson, J., Munizzi, J., and Erhardt, A. M.: Preservation methods for the isotopic composition of dissolved carbon species in non-ideal conditions, Rapid Commun. Mass Sp., 34, e8903, https://doi.org/10.1002/rcm.8903, 2020.
Yang, M. Z., Dong, Q. L., Niu, H. M., Li, J. M., Lin, Z. J., Aslam, M. Z., Wang, X., Li, Z. S., Liu, Y. T., Ma, Y., and Qin, X. J.: Exposure of Salmonella enterica serovar 1,4,[5],12:i:- to benzalkonium chloride leads to acquired resistance to this disinfectant and antibiotics, J. Appl. Microbiol., 134, lxad177, https://doi.org/10.1093/jambio/lxad177, 2023.
Zhang, C., Tezel, U., Li, K. X., Liu, D. F., Ren, R., Du, J. X., and Pavlostathis, S. G.: Evaluation and modeling of benzalkonium chloride inhibition and biodegradation in activated sludge, Water Res., 45, 1238–1246, https://doi.org/10.1016/j.watres.2010.09.037, 2011.
Zhang, C., Cui, F., Zeng, G. M., Jiang, M., Yang, Z. Z., Yu, Z. G., Zhu, M. Y., and Shen, L. Q.: Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment, Sci. Total Environ., 518, 352–362, https://doi.org/10.1016/j.scitotenv.2015.03.007, 2015.
Zhang, J., Quay, P. D., and Wilbur, D. O.: Carbon-isotope fractionation during gas-water exchange and dissolution of CO2, Geochim. Cosmochim. Ac., 59, 107–114, https://doi.org/10.1016/0016-7037(95)91550-D, 1995.